��Ŀ����
�����ǷŴ��ط�����Դ����ҵ����Ҳ���������á�ij��ѧ��ȤС����ʵ�������÷���������������ͭ�ĺϽ���ȡ��������Һ������ͭ���������(Fe2O3)����ʵ�鷽������: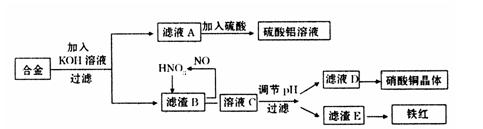
��1����д���ںϽ��м���KOH��Һ�������������ӷ�Ӧ����ʽ��
��
��2������ҺA��ֱ�Ӽ�����������õ���������Һ�лẬ�����ʣ�K2SO4���������һ����������ʵ�鷽������ҺA�Ʊ���������������Һ��������ͼ��ʽ�����Ʊ�����ͼ������ʾ���ڼ�ͷ���·���������Լ���ʵ�������
��3����֪Fe(OH)3������pH��2~3.2����ҺCͨ������pH����ʹFe3+������ȫ��������
���У�������������ҺC��pH���Լ��� ������ţ�
| A��ͭ�� | B����ˮ | C������ͭ | D��������ͭ |
����������ƣ�����Ȼ���
��5����0.1L�Ļ������Һ�У�c(HNO3)=2mol��L-1��c(H2SO4)=3mol��L-1����0.3mol��ͭ���벢��ַ�Ӧ������ͭ�εĻ�ѧʽ�� ������ԭ��n(HNO3��= ��
��1��2Al+2OH-+2H2O=2AlO2-+3H2�� ��2�֣�
��2��4��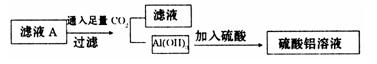
��3��CD ��2�֣�
��4�����ˣ�2�֣�
��5��CuSO4 (2��) 0.2mol(2��)
����

��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ


