��Ŀ����
ʵ��������һƿ��������M�����ǩ�Ұ벿���ѱ���ʴ��ʣ�ಿ��ֻ�ܿ�����Na2S��������ȡ��������M���Ƴ���Һ����ʵ�顣����˵���������
- A.����Һ�м���ϡHCl���������ټ���BaCl2��Һ�������ɰ�ɫ�����������M�϶�ΪNa2SO4
- B.����Һ��ͨ�������CO2�������ɰ�ɫ�����������MΪNa2SiO3
- C.ֻ��NaOHһ���Լ�����֪���ù���M�ijɷ�
- D.ֻ������һ���Լ��Ϳ���֪���ù���M�ijɷ�
���ᱵ������ˮ��Ҳ�������ᣬA��ȷ��̼�������ǿ�ڹ���ģ�B��ȷ������ѡ��AB��֪��C�Ǵ���ģ�D����ȷ�ġ����Դ�ѡC��

����һƿʵ���ҷ����ѾõĿ��ܱ�������Na2SO3���壬Ϊ���о�������ɣ��������ͬѧ�ǽ��е�����̽�����
��ѡ���Լ���ŨH2SO4��ŨHNO3��10%���ᡢ0.1mol/LH2SO4��0.1mol/LHNO3��0.1mol/LBaCl2��0.1mol/LBa(NO3)2��3%H2O2��10%NaOH��Һ������ˮ��Ʒ����Һ��������ѡ��
��1���������
����һ������ȫ����Na2SO3�� �����������ȫ����Na2SO4��
�������� ��
��2�����ʵ�鷽��(��)��ѡ����ͼװ�ý���ʵ�飬��װ�õ��ŵ��� ��
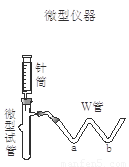
��3������ʵ�飺�����±����ü�Ҫ����д��ʵ�������Ԥ������ͽ��ۡ�
|
ʵ�鲽�� |
Ԥ������ͽ��� |
|
����1��ȡ����������Ʒ�����Թ��У���W��a������ ��b������ ���ý��ܽ�W�������Թ����Ӻ� |
|
|
����2������Ͳ���� ������ͷ�������ԹܵĽ������������Ʒ��ע�����Һ�� |
�� |
|
����3��������Ͳ����������ˮϴ���������� ע�����Թ��� |
�� |
��4����������̽�����������ɵ�����ͨ�뵽����������Ư��Ũ��Һ�У�������ɰ�ɫ��������д���÷�Ӧ�����ӷ���ʽ�� ��