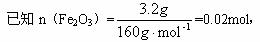��Ŀ����
ij��ѧ��ȤС��Թ���̿������������Ӧ����������ijɷֽ����о���
(1)����÷�Ӧ���������ȫ���Ƕ�����̼��
(2)��Ʒ�������һ�����������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
(3)�������ϣ���������̿��������������Ӧ������������������
(4)ʵ�飺
���������ʵ������ | ��� |
�ٳ�ȡ | ��д��װ���б�����������ƣ� A__________��B__________ |
�ڼ���ǰ����ͨ��һ��������������ĵ��� | ����Ŀ����_______________ |
�ۼн�T�����ɼУ�����һ��ʱ�䣬����ʯ��ˮ����� | �۸�����˵��__________ |
����ȫ��Ӧ����ȴ�����£��Ƶò����ܺ����������Ϊ | �� |
(5)���ݴ����������㣬�μӷ�Ӧ��̼Ԫ������Ϊ_________g,��Ԫ������Ϊ____________g��
(6)���ۣ��������ݴ���������ó�ԭ���費������������_____________________________��
����������Ϊ�о���ʵ���⡣��Ƶ�ʵ��װ�����п�������ʱ�����е���������̿�ۼ���Ӧ���ɵ���������Ӧ����Ӱ��ⶨ������ʼ���ǰ��ͨ��N2�ž�װ���еĿ�����������ʯ��ˮ�����ʱ˵����ʼ��Ӧ������CO2���塣
��֪n(Fe2O3)=![]() =0.02 mol,n(C)=
=0.02 mol,n(C)=![]() ��0.17 mol,��2Fe2O3+
��0.17 mol,��2Fe2O3+![]() 4Fe+3CO2��,
4Fe+3CO2��,
��֪̼�������μӷ�Ӧ����Ԫ��Ϊ��m(O)=0.02 mol��3��![]() n(Fe2O3)��M(C)=
n(Fe2O3)��M(C)= ![]() ��0.02 mol��
��0.02 mol��
�μӷ�Ӧ��̼����������Ϊ
�𰸣�(4)������̨ �Թ�
���ų�װ���еĿ�������ֹO2��̿�ۼ����ɵ�����Ӧ
��̿����Fe2O3��Ӧ������CO2����
(5)0.36 0.96
(6)����������CO2������С��ʵ�ʼ��ٵ�����

ij��ѧ��ȤС��Թ���̿������������Ӧ����������ijɷֽ����о���

��1������÷�Ӧ���������ȫ���Ƕ�����̼��
��2����Ʒ�������һ�����������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
��3���������ϣ���������̿��������������Ӧ������������������
��4��ʵ�飺
| ���������ʵ������ | ��� |
| �ٳ�ȡ3.2 g��������2 g ̿�۾��Ȼ�ϣ�����48.48 g�IJ������У�����ͼװ������ | ��д��װ���б�����������ƣ� A__________��B__________ |
| �ڼ���ǰ����ͨ��һ��������������ĵ��� | ����Ŀ����_______________ |
| �ۼн�T�����ɼУ�����һ��ʱ�䣬����ʯ��ˮ����� | �۸�����˵��__________ |
| ����ȫ��Ӧ����ȴ�����£��Ƶò����ܺ����������Ϊ52.24 g | �� |
(5)���ݴ����������㣬�μӷ�Ӧ��̼Ԫ������Ϊ_________g,��Ԫ������Ϊ____________g��
��6�����ۣ��������ݴ���������ó�ԭ���費������������_____________________________��