��Ŀ����
��1��������ء���������������ɵĻ����Һ�У�c��H+��=0.1 mol?L-1��c��Al3+��=0.4 mol?L-1��c��SO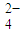 ��=0.8 mol?L-1����c��K+��Ϊ
��=0.8 mol?L-1����c��K+��Ϊ ��2����״���£���33.6L��NH3�ܽ���ˮ���2L����Һ������Һ�����ʵ���Ũ��Ϊ mol/L
��3����״���£���aL NH3�к��е���ԭ����Ϊb�����ӵ�����NA= ���ú���a������b����ʽ������ʾ��
���𰸡���������1�����ݵ���غ���c��H+��+3c��Al3+��+c��K+��=2c��SO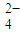 �����ݴ˼��㣻
�����ݴ˼��㣻
��2������n=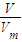 ����33.6L��NH3�����ʵ������ٸ���c=
����33.6L��NH3�����ʵ������ٸ���c=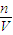 ���㰱ˮ�����ʵ���Ũ�ȣ�
���㰱ˮ�����ʵ���Ũ�ȣ�
��3������n=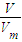 ����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤��������
����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤��������
����⣺��1�����ݵ���غ���c��H+��+3c��Al3+��+c��K+��=2c��SO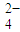 ��������
��������
0.1 mol?L-1+3×0.4 mol?L-1+c��K+��=2×0.8 mol?L-1����c��K+��=0.3mol/L��
�ʴ�Ϊ��0.3mol/L��
��2����״���£�33.6L��NH3�����ʵ���Ϊ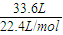 =1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ
=1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ =0.75mol/L��
=0.75mol/L��
�ʴ�Ϊ��0.75��
��3����״���£���aL NH3�����ʵ���Ϊ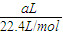 =
=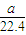 mol�����е���ԭ����Ϊb����
mol�����е���ԭ����Ϊb����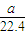 mol×3×NA=b�����NA=
mol×3×NA=b�����NA=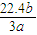 mol-1��
mol-1��
�ʴ�Ϊ��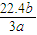 mol-1��
mol-1��
���������⿼�����ʵ���Ũ�ȵ��йؼ��㡢����٤���������йؼ���ȣ��ѶȲ���ע�⣨1���е���ʻ����Һ������Ũ�Ⱦ������õ���غ���м��㣮
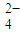 �����ݴ˼��㣻
�����ݴ˼��㣻��2������n=
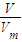 ����33.6L��NH3�����ʵ������ٸ���c=
����33.6L��NH3�����ʵ������ٸ���c=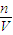 ���㰱ˮ�����ʵ���Ũ�ȣ�
���㰱ˮ�����ʵ���Ũ�ȣ���3������n=
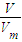 ����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤��������
����aL��NH3�����ʵ�������������Hԭ�ӵ����ʵ������ٸ���N=nNA���㰢��٤������������⣺��1�����ݵ���غ���c��H+��+3c��Al3+��+c��K+��=2c��SO
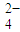 ��������
��������0.1 mol?L-1+3×0.4 mol?L-1+c��K+��=2×0.8 mol?L-1����c��K+��=0.3mol/L��
�ʴ�Ϊ��0.3mol/L��
��2����״���£�33.6L��NH3�����ʵ���Ϊ
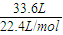 =1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ
=1.5mol������ˮ���2L����Һ���ʰ�ˮ�����ʵ���Ũ��Ϊ =0.75mol/L��
=0.75mol/L���ʴ�Ϊ��0.75��
��3����״���£���aL NH3�����ʵ���Ϊ
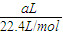 =
=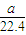 mol�����е���ԭ����Ϊb����
mol�����е���ԭ����Ϊb����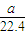 mol×3×NA=b�����NA=
mol×3×NA=b�����NA=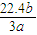 mol-1��
mol-1���ʴ�Ϊ��
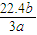 mol-1��
mol-1�����������⿼�����ʵ���Ũ�ȵ��йؼ��㡢����٤���������йؼ���ȣ��ѶȲ���ע�⣨1���е���ʻ����Һ������Ũ�Ⱦ������õ���غ���м��㣮

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
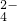 ��=0.8 mol?L-1����c��K+��Ϊ______
��=0.8 mol?L-1����c��K+��Ϊ______