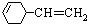��Ŀ����
�밴Ҫ����գ�
��1��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104s2�������ڵ�
��2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵĵ����Ų�ʽΪ
��3����֪����Ԫ�������ڱ��е�λ�ã�д�������������ӵ��Ų�ʽ��Ԫ�ط��ţ�
�ٵ�4���ڢ�B��
�ڵ�5���ڢ�A��
��4����CaC2��C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ
 1mol O22+�к��еĦм���ĿΪ
1mol O22+�к��еĦм���ĿΪ
����Ȳ�������ᷴӦ�ɵñ�ϩ�棨H2C=CH-C��N������ϩ�������̼ԭ�ӹ���ӻ�������
��1��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104s2�������ڵ�
4
4
���ڣ�����B
��B
�壬ds
ds
��Ԫ�أ�Ԫ�ط�����Zn
Zn
��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d54s1�������ڵ������ڣ�d��Ԫ�أ�Ԫ�ط�����Cr
Cr
����2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵĵ����Ų�ʽΪ
[Ar]3d64s2
[Ar]3d64s2
��E+��K��L��M��ȫ������E��Ԫ�ط�����Cu
Cu
�����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1
[Ar]3d104s1
��3����֪����Ԫ�������ڱ��е�λ�ã�д�������������ӵ��Ų�ʽ��Ԫ�ط��ţ�
�ٵ�4���ڢ�B��
Ti��3d64s2
Ti��3d64s2
���ڵ�5���ڢ�A��
I��5s25p5
I��5s25p5
����4����CaC2��C22-��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ


2
2
������Ȳ�������ᷴӦ�ɵñ�ϩ�棨H2C=CH-C��N������ϩ�������̼ԭ�ӹ���ӻ�������
sp�ӻ���sp2 �ӻ�
sp�ӻ���sp2 �ӻ�
�������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ3
3
����������1���۵��ӹ���Ϊ3d104s2��Ӧλ�ڵ�4���ڢ�B�壬�۵��ӹ���Ϊ3d54s1��ӦΪ��4���ڢ�B�壻
��2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵļ۵����Ų�Ϊ3d64s2��ӦΪ��Ԫ�أ�E+��K��L��M��ȫ������ӦΪCuԪ�أ�
��3����4���ڢ�B��ΪTiԪ�أ���5���ڢ�A��ΪIԪ�أ�
��4��O22+�ĵ���ʽ��C22-���ƣ�����O��O������ϼ۲���Ӷ��ж��ӻ����ͣ�
��2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵļ۵����Ų�Ϊ3d64s2��ӦΪ��Ԫ�أ�E+��K��L��M��ȫ������ӦΪCuԪ�أ�
��3����4���ڢ�B��ΪTiԪ�أ���5���ڢ�A��ΪIԪ�أ�
��4��O22+�ĵ���ʽ��C22-���ƣ�����O��O������ϼ۲���Ӷ��ж��ӻ����ͣ�
����⣺��1���۵��ӹ���Ϊ3d104s2��Ӧλ�ڵ�4���ڢ�B�壬ΪZnԪ�أ�λ�����ڱ�ds�����۵��ӹ���Ϊ3d54s1��ӦΪ��4���ڢ�B�壬ΪCrԪ�أ�
�ʴ�Ϊ��4����B��ds��Zn��Cr��
��2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵļ۵����Ų�Ϊ3d64s2��ӦΪ��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d64s2��E+��K��L��M��ȫ������ӦΪCuԪ�أ���̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��[Ar]3d64s2��Cu��[Ar]3d104s1��
��3����4���ڢ�B��ΪTiԪ�أ��������ӵ��Ų�ʽΪ3d24s2����5���ڢ�A��ΪIԪ�أ��������ӵ��Ų�ʽ5s25p5��
�ʴ�Ϊ��Ti��3d64s2��I��5s25p5��
��4����O22+�ĵ���ʽ��C22-���ƣ�����ʽΪ ������O��O�������еĦм���ĿΪ2��
������O��O�������еĦм���ĿΪ2��
�ʴ�Ϊ�� ��2��
��2��
��H2C=CH-C��N��Cԭ�ӷֱ��γ�3���ļ���2���ļ�����̼ԭ�ӹ���ӻ�������sp�ӻ���sp2 �ӻ��������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ3��
�ʴ�Ϊ��sp�ӻ���sp2 �ӻ���3��
�ʴ�Ϊ��4����B��ds��Zn��Cr��
��2��D3+��3d�Dz�Ϊ����������̬ԭ�ӵļ۵����Ų�Ϊ3d64s2��ӦΪ��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d64s2��E+��K��L��M��ȫ������ӦΪCuԪ�أ���̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��[Ar]3d64s2��Cu��[Ar]3d104s1��
��3����4���ڢ�B��ΪTiԪ�أ��������ӵ��Ų�ʽΪ3d24s2����5���ڢ�A��ΪIԪ�أ��������ӵ��Ų�ʽ5s25p5��
�ʴ�Ϊ��Ti��3d64s2��I��5s25p5��
��4����O22+�ĵ���ʽ��C22-���ƣ�����ʽΪ
 ������O��O�������еĦм���ĿΪ2��
������O��O�������еĦм���ĿΪ2���ʴ�Ϊ��
 ��2��
��2����H2C=CH-C��N��Cԭ�ӷֱ��γ�3���ļ���2���ļ�����̼ԭ�ӹ���ӻ�������sp�ӻ���sp2 �ӻ��������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ3��
�ʴ�Ϊ��sp�ӻ���sp2 �ӻ���3��
���������⿼���Ϊ�ۺϣ��漰ԭ�Ӻ�����ӵ��Ų���Ԫ�������ڱ��е�λ�õ��ж��Լ��ӻ����͵�֪ʶ��������ѧ���ķ��������Ŀ��飬�Ѷ��еȣ�ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ

 +2Ag��NH3��2OH
+2Ag��NH3��2OH +2Ag+3NH3+H2O
+2Ag+3NH3+H2O
 ��
��