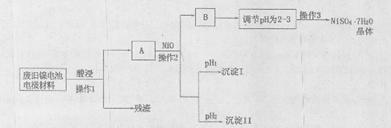
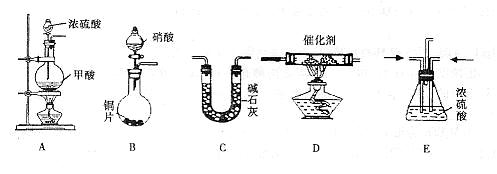
��Ŀ����
I������ʵ��������Լ����淽����ʵ�����¹ʴ�����һ����ȷ���� ������ţ���
E����ʽ�ζ�����ȡ20.00mL�������������Һ��
F���ڽ����к��Ȳⶨʱ��Ϊ��֤ʵ���ȷ�ԣ����ǿ��Բ�ȡ���¾����ʩ��ʹ������ĭ�����ȱ��µ����á�ʹ��ͭ�ʽ�������н��衢ȡ�õļ���Һ�Թ�����������������ʵ��ȡƽ��ֵ��
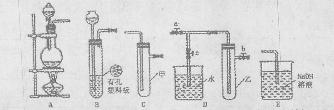
II������β������Ҫ�ɷ�ΪCO2��CO��NOX��NO��NO2���Ļ�������NO���������ռ95%���ϣ��ȡ���������֮һ���������������ϼ�װ����ת�����������ô���ʹCO��NOX������Ӧ��ת��ΪCO2��N2��ijС����ʵ����������ͼ��ʾװ��ģ�������β����CO��NOX�ķ�Ӧ��������Ӧ�����������ɡ�����֪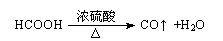 ��
��
�Իش��������⣺
(1)����������˳��Ϊ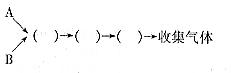
(2)Eװ�õ�������___________________��___________________________________________��
(3)д��D�е�NOX��CO��Ӧ�Ļ�ѧ����ʽ______________________________________��
(4)��B���������ɵ�NOXΪNO��д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ_____________________________ ____________________________��
____________________________��
(5)��ͨ���NOXΪNO��Cװ������8.8g���ռ����������ڱ�״����Ϊ4.48L������Է�������Ϊ28.4�������ռ�����������NO�����ʵ���Ϊ___________________��
(6)ѡ���Ч������������β��ת��Ϊ�����壬�㳹���������β���Ի�����Ӱ�죬����˵���Ƿ���ȷ���������ɣ�___________________________________________��
| A��ʵ�����У�Ũ���ᱣ���ڴ���������ɫϸ���Լ����У� |
| B���Ʊ�������������ʱ��Ӧ��20mL��ˮ����εμ�1~2mL���͵�FeCl3��Һ�����������ȵ�Һ������ĺ��ɫΪֹ�� |
C����ʯ�͵ķ���ʵ���У��¶� �������Һ���У� �������Һ���У� |
| D��������Ũ��Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ�� |
F���ڽ����к��Ȳⶨʱ��Ϊ��֤ʵ���ȷ�ԣ����ǿ��Բ�ȡ���¾����ʩ��ʹ������ĭ�����ȱ��µ����á�ʹ��ͭ�ʽ�������н��衢ȡ�õļ���Һ�Թ�����������������ʵ��ȡƽ��ֵ��
II������β������Ҫ�ɷ�ΪCO2��CO��NOX��NO��NO2���Ļ�������NO���������ռ95%���ϣ��ȡ���������֮һ���������������ϼ�װ����ת�����������ô���ʹCO��NOX������Ӧ��ת��ΪCO2��N2��ijС����ʵ����������ͼ��ʾװ��ģ�������β����CO��NOX�ķ�Ӧ��������Ӧ�����������ɡ�����֪
 ��
��
�Իش��������⣺
(1)����������˳��Ϊ
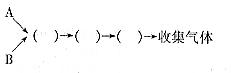
(2)Eװ�õ�������___________________��___________________________________________��
(3)д��D�е�NOX��CO��Ӧ�Ļ�ѧ����ʽ______________________________________��
(4)��B���������ɵ�NOXΪNO��д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ_____________________________
 ____________________________��
____________________________��(5)��ͨ���NOXΪNO��Cװ������8.8g���ռ����������ڱ�״����Ϊ4.48L������Է�������Ϊ28.4�������ռ�����������NO�����ʵ���Ϊ___________________��
(6)ѡ���Ч������������β��ת��Ϊ�����壬�㳹���������β���Ի�����Ӱ�죬����˵���Ƿ���ȷ���������ɣ�___________________________________________��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
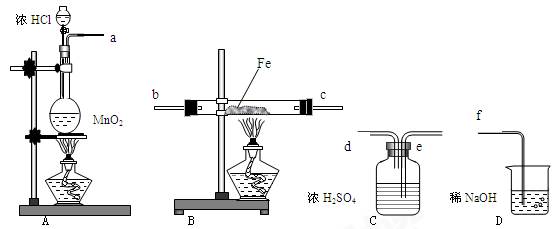
 ���˵�����ɣ� ��
���˵�����ɣ� ��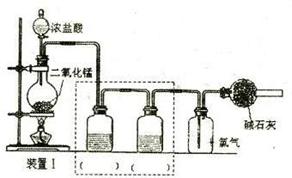
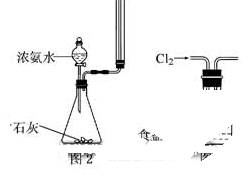
 �ܶ�Ϊ1.84 g��mL-1��_____________mL��
�ܶ�Ϊ1.84 g��mL-1��_____________mL�� ��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£�
��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£� �����ո����Բ��ӣ���
�����ո����Բ��ӣ��� ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���