��Ŀ����
����ѧ��ѡ��5���л���ѧ������
��Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij�о�С���������̿�(��Ҫ�ɷ�ΪMnO2��������������������ͭ�����Ƚ���������)���������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2����Ӧ������ʡ�ԣ���
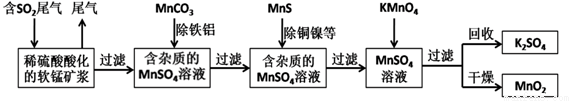
��ش��������⣺
(1)���������ж����Ƶ����˲�����ʵ���ҽ��й��˲���ʱ��Ҫ�õ��Ĺ����������в��������ձ���_______________�����в�������������___________________��
(2)��MnCO3�ܳ�ȥ��Һ�е�Al3+��Fe3+����ԭ����_____________(ֻ�����ֱ���)
(3)��ҵ�ϲ��õ��K2MnO4ˮ��Һ�ķ���������KMnO4�����ж��Ե缫��������������������д�������ĵ缫��Ӧʽ__________________��
(4)���и����Լ��У���ȷ�ⶨһ�����ȼúβ����SO2��������__________��(����)
a��NaOH��Һ����̪��Һ b��ϡH2SO4�ữ��KMnO4��Һ
c����ˮ��������Һ d����ˮ����̪��Һ
(5)������SO2��ϡ�����ữ�����̿�Ӧ�Ļ�ѧ����ʽΪ_______________����֪������SO2Ũ��Ϊ6.4g/m3�����̿�SO2�������ʿɴ�90%������1000m3ȼúβ�����ɵõ������̾���(MnSO4��H2O����Է�������Ϊ169)����Ϊ_________________kg(�������3 λ��Ч����)��
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�

 2NO2(g) ��H=+24.4KJ/mol
2NO2(g) ��H=+24.4KJ/mol v��(N2O4)=2v��(NO2) b����ϵ��ɫ����
v��(N2O4)=2v��(NO2) b����ϵ��ɫ���� CO(g)+3H2(g)������3min����Ӧ�ﵽƽ�⡣��֪ƽ��ʱc(CH4
CO(g)+3H2(g)������3min����Ӧ�ﵽƽ�⡣��֪ƽ��ʱc(CH4 )=0.5mol/L
)=0.5mol/L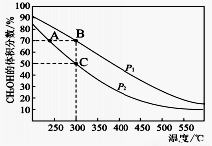
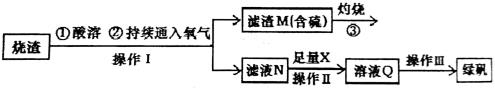
 ��Һ������ɫ����___________
��Һ������ɫ����___________ �����
�����