��Ŀ����
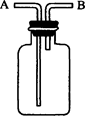
��10�֣���ͼ��������������ˮ����Ȫʵ��װ�ã�ʵ����ɺ���ƿ��Ŀռ䱻��Ϊ�������֣�A��B �������ʵ�����ݰ�Ҫ����գ�
�������ʵ�����ݰ�Ҫ����գ�
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
 �������ʵ�����ݰ�Ҫ����գ�
�������ʵ�����ݰ�Ҫ����գ�
��1��A�е���Ҫ�ɷ��� ��
��2��B����Һ��Ϊ��ˮ���ѷ�̪���백ˮ�У���Һ�� ɫ������Ϊ �������ӷ���ʽ��ʾ����
��3���Լ��ĵμ�˳��ͬ����ʱ�������ͬ������
�ٰѰ�ˮ����Al2(SO4)3��Һ�У��ڰ�Al2(SO4)3��Һ���백ˮ�У� �١��ڵ�ʵ�������Ƿ���ͬ (���ͬ����ͬ��)��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�ڵ����ӷ���ʽ ��
��4���Ѱ�ˮ�μӵ�FeSO4��Һ�е�����Ϊ
��
��7�֣���1��������N2��O2����2����ɫ�� NH3��H2O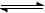 NH4+ + OH-[
NH4+ + OH-[
��3���� Al2(SO4)3+6NH3��H2O + 6H2O��2Al(OH)3��+3(NH4)2SO4
��Al3++3NH3��H2O ��Al(OH)3��+3NH4+
��4�������ɰ�ɫ�������ú��ɻ���ɫ������ɺ��ɫ��
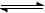 NH4+ + OH-[
NH4+ + OH-[��3���� Al2(SO4)3+6NH3��H2O + 6H2O��2Al(OH)3��+3(NH4)2SO4
��Al3++3NH3��H2O ��Al(OH)3��+3NH4+
��4�������ɰ�ɫ�������ú��ɻ���ɫ������ɺ��ɫ��
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ