��Ŀ����
��2008?���죩��Ȼ�������������е�֧����ҵ֮һ������Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT��

��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ��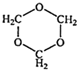
 ��B��ͬ���칹�����������Ǿ������ƽṹ����
��B��ͬ���칹�����������Ǿ������ƽṹ����
 ����д�ṹ��ʽ��
����д�ṹ��ʽ��
��2��F�Ľṹ��ʽ��
��3����A��D����E�ķ�Ӧ����ʽΪ
��4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ��

��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ��




��2��F�Ľṹ��ʽ��
HO-CH2-CH2-CH2-CH2-OH
HO-CH2-CH2-CH2-CH2-OH
��PBT������
��
���л��߷��ӻ������3����A��D����E�ķ�Ӧ����ʽΪ
HC��CH+2HCHO
HOCH2C��CCH2OH
| �� |
HC��CH+2HCHO
HOCH2C��CCH2OH
���䷴Ӧ����Ϊ| �� |
�ӳɷ�Ӧ
�ӳɷ�Ӧ
����4��E��ͬ���칹��G���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH
CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH
����������1���״���Cu�����������������������������ɼ�ȩ��HCHO������AΪHCHO�����A��B�ķ���ʽ��֪��BΪ3���Ӽ�ȩ�γɣ�B���ӽṹΪ��Ԫ��״�����Ӧ��3���Ӽ�ȩ֮��ļӳɷ�Ӧ����B��
B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ��
��2����E��F��ת����F����ʽ��֪��E�к���4��Cԭ�ӣ���HCHO+D��E���ٸ�����֪��Ӧ��Ϣ��֪���÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ��DΪHC��CH��EΪHOCH2-C��C-CH2OH��E�����������ӳɷ�Ӧ����F��F���Ԫ����ͨ��������Ӧ�γɸ߾���PBT��
��3���ɣ�2���з�����֪����A��D����E�ķ�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ����HOCH2-C��C-CH2OH��
��4��HOCH2-C��C-CH2OH��ͬ���칹��G���ܷ���������Ӧ�������в���ȩ��-CHO��-OOCH����ʹ��ˮ��ɫ�����в����ͼ�����ˮ���Ҳ����̼ԭ�������ȣ������������ʺ���1��C=C˫����1�����������γɸ��������Ậ��3��̼ԭ�ӣ���Ϊ�״����ݴ��ж�G�Ľṹʽ����д����ʽ��
B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ��
��2����E��F��ת����F����ʽ��֪��E�к���4��Cԭ�ӣ���HCHO+D��E���ٸ�����֪��Ӧ��Ϣ��֪���÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ��DΪHC��CH��EΪHOCH2-C��C-CH2OH��E�����������ӳɷ�Ӧ����F��F���Ԫ����ͨ��������Ӧ�γɸ߾���PBT��
��3���ɣ�2���з�����֪����A��D����E�ķ�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ����HOCH2-C��C-CH2OH��
��4��HOCH2-C��C-CH2OH��ͬ���칹��G���ܷ���������Ӧ�������в���ȩ��-CHO��-OOCH����ʹ��ˮ��ɫ�����в����ͼ�����ˮ���Ҳ����̼ԭ�������ȣ������������ʺ���1��C=C˫����1�����������γɸ��������Ậ��3��̼ԭ�ӣ���Ϊ�״����ݴ��ж�G�Ľṹʽ����д����ʽ��
����⣺��1���״��������ɼ�ȩ��HCHO�������A��B�ķ���ʽ��֪��BΪ3���Ӽ�ȩ�γɣ�B���ӽṹΪ��Ԫ��״�����Ӧ��3���Ӽ�ȩ֮��ļӳɷ�Ӧ����B����B�Ľṹ��ʽΪ�� ��B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ����ͬ���칹��Ľṹ��ʽΪ
��B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ����ͬ���칹��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2����E��F��ת����F����ʽ��֪��E�к���4��Cԭ�ӣ���HCHO+D��E���ٸ�����֪��Ӧ��Ϣ��֪���÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ��DΪHC��CH��EΪHOCH2-C��C-CH2OH��E�����������ӳɷ�Ӧ����F����F�ṹ��ʽΪHO-CH2-CH2-CH2-CH2-OH��F���Ԫ����ͨ��������Ӧ�γɸ߾���PBT��PBT�������࣬
�ʴ�Ϊ��HO-CH2-CH2-CH2-CH2-OH������
��3���ɣ�2���з�����֪���÷�ӦΪ�÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ����HOCH2-C��C-CH2OH����A��D����E�ķ�Ӧ����ʽΪ��HC��CH+2HCHO
HOCH2C��CCH2OH��
�ʴ�Ϊ��HC��CH+2HCHO
HOCH2C��CCH2OH���ӳɷ�Ӧ��
��4��HOCH2-C��C-CH2OH��ͬ���칹��G���ܷ���������Ӧ�������в���ȩ��-CHO��-OOCH����ʹ��ˮ��ɫ�����в����ͼ�����ˮ���Ҳ����̼ԭ�������ȣ������������ʺ���1��C=C˫����1�����������γɸ��������Ậ��3��̼ԭ�ӣ���Ϊ�״�����G�Ľṹ��ʽΪ��CH2=CH-COOCH3��CH2=CH-COOCH3������������Һ�з���ˮ�ⷽ��ʽΪ��
CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH��
�ʴ�Ϊ��CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH��
 ��B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ����ͬ���칹��Ľṹ��ʽΪ
��B��ͬ���칹���������ǽṹ���ƣ����칹���к���1��-CHO��2���ǻ�-OH��Ϊ��״�ṹ����ͬ���칹��Ľṹ��ʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
����2����E��F��ת����F����ʽ��֪��E�к���4��Cԭ�ӣ���HCHO+D��E���ٸ�����֪��Ӧ��Ϣ��֪���÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ��DΪHC��CH��EΪHOCH2-C��C-CH2OH��E�����������ӳɷ�Ӧ����F����F�ṹ��ʽΪHO-CH2-CH2-CH2-CH2-OH��F���Ԫ����ͨ��������Ӧ�γɸ߾���PBT��PBT�������࣬
�ʴ�Ϊ��HO-CH2-CH2-CH2-CH2-OH������
��3���ɣ�2���з�����֪���÷�ӦΪ�÷�ӦΪ2���Ӽ�ȩ����Ȳ�����ӳɷ�Ӧ����HOCH2-C��C-CH2OH����A��D����E�ķ�Ӧ����ʽΪ��HC��CH+2HCHO
| �� |
�ʴ�Ϊ��HC��CH+2HCHO
| �� |
��4��HOCH2-C��C-CH2OH��ͬ���칹��G���ܷ���������Ӧ�������в���ȩ��-CHO��-OOCH����ʹ��ˮ��ɫ�����в����ͼ�����ˮ���Ҳ����̼ԭ�������ȣ������������ʺ���1��C=C˫����1�����������γɸ��������Ậ��3��̼ԭ�ӣ���Ϊ�״�����G�Ľṹ��ʽΪ��CH2=CH-COOCH3��CH2=CH-COOCH3������������Һ�з���ˮ�ⷽ��ʽΪ��
CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH��
�ʴ�Ϊ��CH2=CH-COOCH3+NaOH��CH2=CH-COONa+CH3OH��
���������⿼���л�����ƶ���ϳɣ���������л���ķ���ʽ�뷴Ӧ��Ϣ�����жϣ��Ѷ��еȣ��ܽϺõĿ��鿼�����Ķ�����ѧ������˼ά���������ȵ����ͣ�

��ϰ��ϵ�д�
�����Ŀ
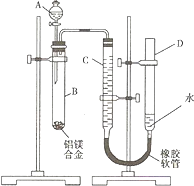 ��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
