��Ŀ����
(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
| ���� | �������� | �Ȼ��� |
| 1�����������µ��ȶ��� | ��ʱ�ֽ�ΪNO��NO2 | ��ʱ���ֽ� |
| 2���۵� | 271�� | 801�� |
| 3������ʱ���ܽ�� | Լ80g | Լ35g |
������ݱ�����Ϣ���һ�ּ���NaNO2��NaCl�ķ�����д���IJ������̡�����ͽ��ۣ�
�ڵ��������в���ȱ�ٵ� ��ѡ���������������Ԫ�ء�
��ʳ���м����Ԫ������Ч��ֹȱ������ļ�������ǰ��ʳ���м���⻯�أ�KI��������һ�����ʧԼ92%������ʳ���м������أ�KIO3��������ͬ�����µ����ʧԼ7%��ʳ���м���⻯�صĵ���ʧ�ʸߵ�ԭ���� ��������ĸ����
a���⻯�ر���������ɵ⻯�صĵ���ʧ�ʸߡ�
b���⻯����������ɵ⻯�صĵ���ʧ�ʸߡ�
c���⻯����ʳ���еijɷַ�����Ӧ����ɵ⻯�صĵ���ʧ�ʸߡ�
����ȡ��ˮ�еĵ�ʱ��һ��ѡ�õ��Լ��ǣ�����ĸ�� ��
A���ƾ� B�����Ȼ�̼ C������
�ݿ��������ữ�ĵ⻯�غ͵�����Һ����ʳ���еĵ���ء���Ӧ��ѧ����ʽΪ��
5KI��KIO3 + 6HCl == 6KCl + 3I2 +3H2O����Ӧ��������
����֪����������ֽ⣬����Ϊ���õ���ؼӵ��ν������ʱӦע��ʲô���⣿ ��
�����ࡢ��֬�������ʶ�����������Ӫ�����ʡ�
����֬�������������ø��������ˮ��Ϊ��֬����� ��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
�ڰ���������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�������NH2���� ��д�ṹ��ʽ���������й��ж�ʮ���ְ����ᣬ������������_____����ܡ����ܡ����ϳɵİ������Ϊ������谱���ᡣ
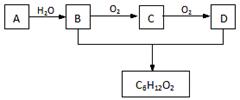
�۵����ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ�� ��
��ʳ�� ��֬�� ��������
�Ƣٺ����𰸾����֢�����1�֣���a��2�֣� ��B ��1�֣�����Һ����ɫ��
�������Ժ����ʱ�ټ���
�Ǣٸ��ͻ��������1�֣� ���Ȼ� ���� ��C6H12O6+6O2=6CO2+6H2O
����:

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
| ���� | �������� | �Ȼ��� |
| 1�����������µ��ȶ��� | ��ʱ�ֽ�ΪNO��NO2 | ��ʱ���ֽ� |
| 2���۵� | 271�� | 801�� |
| 3������ʱ���ܽ�� | Լ80g | Լ35g |
�ڵ��������в���ȱ�ٵ� ��ѡ���������������Ԫ�ء�
��ʳ���м����Ԫ������Ч��ֹȱ������ļ�������ǰ��ʳ���м���⻯�أ�KI��������һ�����ʧԼ92%������ʳ���м������أ�KIO3��������ͬ�����µ����ʧԼ7%��ʳ���м���⻯�صĵ���ʧ�ʸߵ�ԭ���� ��������ĸ����
a���⻯�ر���������ɵ⻯�صĵ���ʧ�ʸߡ�
b���⻯����������ɵ⻯�صĵ���ʧ�ʸߡ�
c���⻯����ʳ���еijɷַ�����Ӧ����ɵ⻯�صĵ���ʧ�ʸߡ�
����ȡ��ˮ�еĵ�ʱ��һ��ѡ�õ��Լ��ǣ�����ĸ�� ��
A���ƾ� B�����Ȼ�̼ C������
�ݿ��������ữ�ĵ⻯�غ͵�����Һ����ʳ���еĵ���ء���Ӧ��ѧ����ʽΪ��
5KI��KIO3 + 6HCl ="=" 6KCl + 3I2 + 3H2O����Ӧ��������
����֪����������ֽ⣬����Ϊ���õ���ؼӵ��ν������ʱӦע��ʲô���⣿ ��
�����ࡢ��֬�������ʶ�����������Ӫ�����ʡ�
����֬�������������ø��������ˮ��Ϊ��֬����� ��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
�ڰ���������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�������NH2���� ��д�ṹ��ʽ���������й��ж�ʮ���ְ����ᣬ������������_____����ܡ����ܡ����ϳɵİ������Ϊ������谱���ᡣ
�۵����ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ�� ��
(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
|
���� |
�������� |
�Ȼ��� |
|
1�����������µ��ȶ��� |
��ʱ�ֽ�ΪNO��NO2 |
��ʱ���ֽ� |
|
2���۵� |
271�� |
801�� |
|
3������ʱ���ܽ�� |
Լ80g |
Լ35g |
������ݱ�����Ϣ���һ�ּ���NaNO2��NaCl�ķ�����д���IJ������̡�����ͽ��ۣ�
�ڵ��������в���ȱ�ٵ� ��ѡ���������������Ԫ�ء�
��ʳ���м����Ԫ������Ч��ֹȱ������ļ�������ǰ��ʳ���м���⻯�أ�KI��������һ�����ʧԼ92%������ʳ���м������أ�KIO3��������ͬ�����µ����ʧԼ7%��ʳ���м���⻯�صĵ���ʧ�ʸߵ�ԭ���� ��������ĸ����
a���⻯�ر���������ɵ⻯�صĵ���ʧ�ʸߡ�
b���⻯����������ɵ⻯�صĵ���ʧ�ʸߡ�
c���⻯����ʳ���еijɷַ�����Ӧ����ɵ⻯�صĵ���ʧ�ʸߡ�
����ȡ��ˮ�еĵ�ʱ��һ��ѡ�õ��Լ��ǣ�����ĸ�� ��
A���ƾ� B�����Ȼ�̼ C������
�ݿ��������ữ�ĵ⻯�غ͵�����Һ����ʳ���еĵ���ء���Ӧ��ѧ����ʽΪ��
5KI��KIO3 + 6HCl == 6KCl + 3I2 + 3H2O����Ӧ��������
����֪����������ֽ⣬����Ϊ���õ���ؼӵ��ν������ʱӦע��ʲô���⣿ ��
�����ࡢ��֬�������ʶ�����������Ӫ�����ʡ�
����֬�������������ø��������ˮ��Ϊ��֬����� ��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
�ڰ���������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�������NH2���� ��д�ṹ��ʽ���������й��ж�ʮ���ְ����ᣬ������������_____����ܡ����ܡ����ϳɵİ������Ϊ������谱���ᡣ
�۵����ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ�� ��
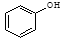 ��
��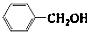 ��Ӧѡ��
___ (����ĸ)��
��Ӧѡ��
___ (����ĸ)��