��Ŀ����
A��J����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��C��F��IΪ��̬���ʣ�E�ڳ�����ΪҺ�壬��E����C��F�ϳɣ�J������ɱ����������

�ش��������⣺
(1)B�Ļ�ѧʽ�� ��E�ĵ���ʽ_________��
��2��д����Ӧ�ߵ����ӷ���ʽ __________________________��
��3����AlCl3��Һ�м�����������B��д����Ӧ�Ļ�ѧ����ʽ_____________________��
��4����PtΪ�缫���μ���������̪��H������Һ������_____________���������������������Һ����ɫ��Ϊ��ɫ����ԭ����_________________ ��

�ش��������⣺
(1)B�Ļ�ѧʽ�� ��E�ĵ���ʽ_________��
��2��д����Ӧ�ߵ����ӷ���ʽ __________________________��
��3����AlCl3��Һ�м�����������B��д����Ӧ�Ļ�ѧ����ʽ_____________________��
��4����PtΪ�缫���μ���������̪��H������Һ������_____________���������������������Һ����ɫ��Ϊ��ɫ����ԭ����_________________ ��
��1��Na2O2��2�֣�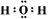
��2��Cl2+2OH��=Cl��+ClO��+H2O
��3��4AlCl3+6Na2O2+6H2O=4Al(OH)3��+12NaCl+3O2��
(4)������1�֣�������������H+�õ����Ӳ���H2���ƻ���ˮ�ĵ���ƽ�⣬�ٽ�ˮ�������룬������Һ��c(OH��)>c(H+)����Һ�ʼ��ԣ���������������Һ��Ϊ��ɫ ��1�֣�
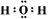
��2��Cl2+2OH��=Cl��+ClO��+H2O
��3��4AlCl3+6Na2O2+6H2O=4Al(OH)3��+12NaCl+3O2��
(4)������1�֣�������������H+�õ����Ӳ���H2���ƻ���ˮ�ĵ���ƽ�⣬�ٽ�ˮ�������룬������Һ��c(OH��)>c(H+)����Һ�ʼ��ԣ���������������Һ��Ϊ��ɫ ��1�֣�
������AΪ���嵥�ʣ�BΪ����ɫ��ĩ����AΪNa��BΪNa2O2����ôCΪO2��E�ڳ�����ΪҺ�壬ӦΪH2O����FΪH2�� DΪNaOH��H��ˮ��Һ��ͨ�������·�Ӧ����H2��I��NaOH��ӦΪ��ⱥ��ʳ��ˮ�ķ�Ӧ����IΪCl2����֪GΪHCl�� HΪNaCl��Cl2��NaOH��Ӧ����NaCl��H2O��NaClO����JΪNaClO����
(1)B�Ļ�ѧʽ��Na2O2��E�ĵ���ʽ
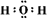
��2��Cl2+2OH��=Cl��+ClO��+H2O
��3��4AlCl3+6Na2O2+6H2O=4Al(OH)3��+12NaCl+3O2��
(4) 2Cl�D��2H2O
 2OH�D��H2����Cl2��OH�D����������ⱥ��ʳ��ˮ��������ӦΪ��2Cl��-2e��=Cl2��������ӦΪ2H��+2e��=H2������������H���õ����Ӳ���H2���ƻ���ˮ�ĵ���ƽ�⣬�ٽ�ˮ�������룬������Һ��c��OH������c��H��������Һ�ʼ��ԣ���������������Һ��Ϊ��ɫ
2OH�D��H2����Cl2��OH�D����������ⱥ��ʳ��ˮ��������ӦΪ��2Cl��-2e��=Cl2��������ӦΪ2H��+2e��=H2������������H���õ����Ӳ���H2���ƻ���ˮ�ĵ���ƽ�⣬�ٽ�ˮ�������룬������Һ��c��OH������c��H��������Һ�ʼ��ԣ���������������Һ��Ϊ��ɫ��Ϊ������������H+�õ����Ӳ���H2���ƻ���ˮ�ĵ���ƽ�⣬�ٽ�ˮ�������룬������Һ��c(OH��)>c(H+)����Һ�ʼ��ԣ���������������Һ��Ϊ��ɫ��

��ϰ��ϵ�д�
�����Ŀ
 ��2��������������ˮ����ʱ��������ɱ�����õ������� ����������������ˮ�����г�ζ���������û����ܶ������DZ��Σ����ClO2��һ������ɱ��Ч�ʸߡ�������ȾС��ˮ����������ҵ�Ͽ���SO2��NaClO3��Һ��Ӧ�Ƶã��÷�Ӧ���ӷ���ʽ�� ��
��2��������������ˮ����ʱ��������ɱ�����õ������� ����������������ˮ�����г�ζ���������û����ܶ������DZ��Σ����ClO2��һ������ɱ��Ч�ʸߡ�������ȾС��ˮ����������ҵ�Ͽ���SO2��NaClO3��Һ��Ӧ�Ƶã��÷�Ӧ���ӷ���ʽ�� ��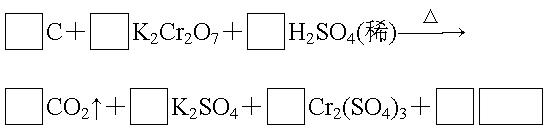
 ʢ����ʳ��ˮ��ϴ��ƿ
ʢ����ʳ��ˮ��ϴ��ƿ