题目内容
(8分)对下图中电极加以必要的连接并填空:
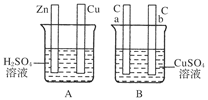
(1)在A图中,使铜片上冒气泡。请加以必要连接,则连接后的装置叫________。电极反应式:
锌板:_______________________________________________________________;
铜板:______________________________________________________________。
(2)在B图中,使a极析出铜,则b极析出________。加以必要的连接后,该装置叫________。电极反应式,a极:________;b极:________。经过一段时间后,停止反应并搅匀溶液,溶液的pH________(填“升高”、“降低”或“不变”)。
(3)将A、B图中Cu板与a极、Zn板与b极用导线连接,则连接后的A装置叫________,B装置叫________。Zn板为________极,a极为________极。A图发生的总反应的化学方程式为__________________,B图发生的总反应的离子方程式为
________________________________________________________________________。

(1)在A图中,使铜片上冒气泡。请加以必要连接,则连接后的装置叫________。电极反应式:
锌板:_______________________________________________________________;
铜板:______________________________________________________________。
(2)在B图中,使a极析出铜,则b极析出________。加以必要的连接后,该装置叫________。电极反应式,a极:________;b极:________。经过一段时间后,停止反应并搅匀溶液,溶液的pH________(填“升高”、“降低”或“不变”)。
(3)将A、B图中Cu板与a极、Zn板与b极用导线连接,则连接后的A装置叫________,B装置叫________。Zn板为________极,a极为________极。A图发生的总反应的化学方程式为__________________,B图发生的总反应的离子方程式为
________________________________________________________________________。
(1)原电池 Zn-2e-===Zn2+
2H++2e-===H2↑
(2)O2 电解池 2Cu2++4e-===2Cu
4OH--4e-===2H2O+O2↑
降低 装置连接如图:

(3)原电池 电解池 负 阳
Zn+H2SO4===ZnSO4+H2↑
2Cu2++2H2O2Cu+O2↑+4H+
2H++2e-===H2↑
(2)O2 电解池 2Cu2++4e-===2Cu
4OH--4e-===2H2O+O2↑
降低 装置连接如图:

(3)原电池 电解池 负 阳
Zn+H2SO4===ZnSO4+H2↑
2Cu2++2H2O2Cu+O2↑+4H+
自发的氧化还原反应可设计成原电池,非自发的氧化还原反应可设计成电解池。
(1)A池为自发反应,将两极用导线连接即可构成原电池,锌为负极,铜为正极。
(2)B中使a极析出铜,则需电解,将a极连接电源负极,则b极为阳极,可析出O2,反应后溶液中生成H2SO4,pH降低。
(3)按题意将两池连接,则A池为原电池,B池为电解池,a极为阳极,b极为阴极。
(1)A池为自发反应,将两极用导线连接即可构成原电池,锌为负极,铜为正极。
(2)B中使a极析出铜,则需电解,将a极连接电源负极,则b极为阳极,可析出O2,反应后溶液中生成H2SO4,pH降低。
(3)按题意将两池连接,则A池为原电池,B池为电解池,a极为阳极,b极为阴极。

练习册系列答案
 智趣暑假温故知新系列答案
智趣暑假温故知新系列答案
相关题目