��Ŀ����
�ɶ�����Ԫ����ɵij�������A��B��C��D��E��X������ͼת����ϵ(����������ͷ�Ӧ������ȥ)����
A��X��Na2CO3��CΪ�����Լ��ķǼ��Է��ӣ���Aһ������������D��E����Ӧ
B��A�ǵ��ʣ�B��D�ķ�Ӧ��OH����HCO3-��H2O��CO32-����Eһ���ܻ�ԭFe2O3
C��X��O2����C��DĦ���������16����Aһ��ΪMg3N2
D��DΪ��ɫ��������AĦ��������ȣ���Xһ��������
BD
�������������A.��AΪNO2��������Ӧ3NO2+H2O=2HNO3+NO��B��HNO3��EΪNO��Na2CO3+ 2 HNO3="2Na" NO3+H2O+ CO2����C��CO2, Na2CO3��CO2+H2O=2NaHCO3.���Aѡ�����B��A�ǵ��ʣ�B��D�ķ�Ӧ��OH����HCO3-��H2O��CO32-����A�ǻ��õĽ�������Na��BΪ�NaOH��EΪH2��X��CO2��C��CO32-��D��HCO3-��E�����ʼ�ķ�ӦΪ2Na+2H2O=2Na++OH-+H2����2OH-��CO2= CO32-+H2O�� CO32-��CO2+H2O=2HCO3-�� Fe2O3+3H2 2Fe+ 3H2O.��ȷ��C.��AΪAl2S3,B��H2S��E��Al(OH)3��C��SO2��D��SO3��������ϵҲ����������D.AΪNa2O2,BΪNaOH��EΪO2��X��AlCl3,CΪNaAlO2��DΪAl(OH)3.������Ӧ��2Na2O2+2H2O="4NaOH+" O2����4NaOH+ AlCl3="3NaCl+" NaAlO2+2H2O��3NaAlO2+ AlCl3+6H2O= 2Al(OH)3��+3NaCl��
2Fe+ 3H2O.��ȷ��C.��AΪAl2S3,B��H2S��E��Al(OH)3��C��SO2��D��SO3��������ϵҲ����������D.AΪNa2O2,BΪNaOH��EΪO2��X��AlCl3,CΪNaAlO2��DΪAl(OH)3.������Ӧ��2Na2O2+2H2O="4NaOH+" O2����4NaOH+ AlCl3="3NaCl+" NaAlO2+2H2O��3NaAlO2+ AlCl3+6H2O= 2Al(OH)3��+3NaCl��
���㣺����Ԫ�ؼ���������ת�����ƶϵ�֪ʶ��

����˵����ȷ����
A������ij������Һ��C1����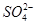 ��ѡ���Լ���˳���ǹ���Ba��NO3��2��Һ��AgNO3��Һ ��ѡ���Լ���˳���ǹ���Ba��NO3��2��Һ��AgNO3��Һ |
| B����CO2��SO2�������ֱ�ͨ��BaC12��Һ��Ba��NO3��2��Һ�У����ն��г������� |
| C�������ۼ���FeCl3��CuCl2�����Һ�У���ַ�Ӧ��ʣ��Ĺ����б����� |
| D���ü��ȷֽ�ķ����ɽ�NH4Cl�����Ca��OH��2����Ļ������� |
����ʵ������������ȷ����
| A��HCl��NH3�����д����İ������� |
| B���ýྻ����պȡNa2SO4��Һ���ջ������ɫ |
| C��FeCl2��Һ����ɫ��KSCN��Һ��ϣ���Һ�ʺ�ɫ |
| D��SO2ͨ��Ʒ����Һ����ɫ��ȥ���ټ��ȣ���ɫ���ٳ��� |
�±��г�ȥ����Ӧѡ�õ��Լ������������ȷ����(����)
| ѡ�� | ���� | ���� | ������Ӧѡ�õ��Լ���������� |
| A | KNO3��Һ | KOH | ����FeCl3��Һ�������� |
| B | FeCl3��Һ | FeCl2 | �����Թ���˫��ˮ����� |
| C | CO | O2 | ͨ�����ȵ�ͭ�����ռ����� |
| D | K2CO3���� | NaHCO3 | ����������� |
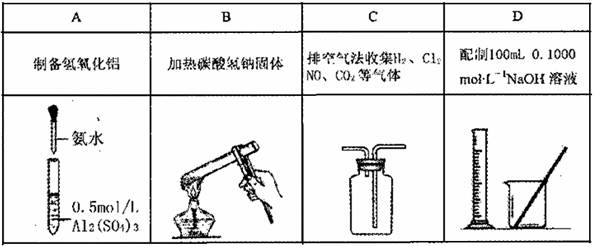
������ͼ��ʾװ����ȡ���������ռ���������( )
| A��п��ϡ���ᷴӦ��һ������ |
| B������������Ũ���ᷴӦ�ƶ������� |
| C������ϡ���ᷴӦ������ |
| D��Ũ��ˮ����ʯ�ҷ�Ӧ��ȡ���� |
�������ʵ�ת���ڸ�����������ʵ�ֵ���( )
| A���٢ۢ� | B���ڢۢ� | C���ڢܢ� | D���٢ܢ� |
�����й�ʵ���������ȷ���� (����)
| A���������ƹ��屣����������Ƥ����ϸ��ƿ�� |
| B����SO2ͨ��Ʒ����Һ��KMnO4��Һ����ʹ������ɫ������֤��SO2��Ư���� |
| C��������ʯӢ�������ۻ��������� |
| D����ʪ���pH��ֽ�ⶨϡ�����pH |