��Ŀ����
��1������Ŀ��Ϣ��֪����Ӧ����
��2������ķ���ʽΪH3BO3����֪H��O�ɼ���������ӽṹʽΪ��
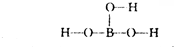
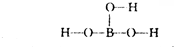
��3���о��������ڴ��������£�Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������дﵽ8�����ȶ��ṹ��������֪0.01mol������Ա�20mL 0.5mol?L-1NaOH��Һǡ����ȫ�кͣ��ݴ��Ʋ�������ˮ��Һ�г����Ե�ԭ���ǣ������ӷ���ʽ��ʾ��
��4������ͼ״���Ũ������ڵ������£������ɻӷ�������������д��������ȫ�����ķ���ʽ��
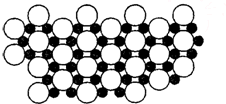
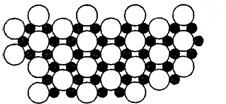
��5����ѧ�ҷ�����þ��39Kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������У�
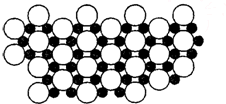
��ͼ�Ǹþ����ۿռ���ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϣ�������ͼȷ����þ�Ļ�ѧʽΪ
��2���������ɷǽ���Ԫ�ع��ɵĹ��ۻ���������ڲ�ȫΪ���۵�����
��3��������ˮ��Һ���ܵ���������Ӷ������ԣ��ܺ��������Ʒ�Ӧ��
��4��������Ӧ��ʵ���ǣ�����ǻ������⣬Ȼ����������ˮ��
��5��1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�1��Mgԭ��Ϊ6��Bԭ�ӹ��ã����Լ�����ԭ�Ӻ�þԪ�Եø���֮�ȣ�
��2������Bԭ�����3�����ӣ���������H��O�ɼ�����H��O�γ��ǻ����ǻ���ԭ��������Bԭ���ϣ�����ÿ���ǻ������ṩ1��������Bԭ�ӹ��ã��������ṹ��ʽΪ��B��OH��3���ṹʽΪ��
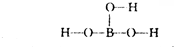 ���ʴ�Ϊ��
���ʴ�Ϊ��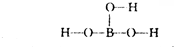 ��
����3��������Bԭ������ȱ����ԭ�ӣ�����ˮ��Һ�п��Խ��ˮ����������������ƻ�ˮ�ĵ���ƽ���ʹ��Һ�����ԣ��ʴ�Ϊ��B��OH��3+H2O=B��OH��4-+H+��B��OH��3+OH-=B��OH��4-��
��4����������Ӧ������������ǻ������������е��ǻ���-OH��״��е���ԭ�ӽ������ˮ���ʴ�Ϊ��B��OH��3+3CH3OH�TB��OCH3��3+3H2O��
��5������ͶӰ��֪��1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�������һ��Mgԭ�ӵ�Bԭ��Ϊ
| 1 |
| 3 |
| 1 |
| 6 |
| 1 |
| 6 |
| 1 |
| 3 |

 �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| |||||||||||||||||||||||||||