��Ŀ����
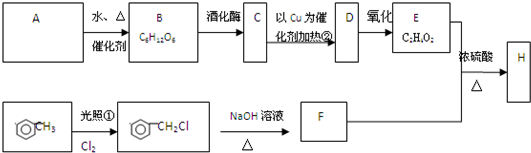
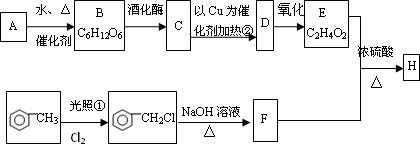
�Ե���AΪԭ�Ͽ����Ƶ���������ζ��H���ְ����·�ʽ���кϳɸ����ϣ�������A��B��C��D��E��F��Ϊ�л��ͬʱ������ijЩ������ʡ�ԣ�
��֪��RCH2Cl��NaOH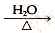 RCH2OH��NaCl
RCH2OH��NaCl

��ش��������⣺
��1��A�Ļ�ѧʽΪ______��B������Ϊ______��
��2��д���٢ڵķ�Ӧ���ͣ���______����______��
��3��C��D�Ļ�ѧ����ʽΪ______��
��4��F+E��H�Ļ�ѧ����ʽΪ______��
��5��д��C������ͬ���칹��Ľṹ��ʽ______��______��
��11�֣���1����C6H10O5��n��1�֣������ǣ�1�֣� ��2��ȡ�� ��1�֣� ������1�֣�
��3�� ��2�֣�
��2�֣�
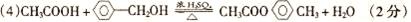
��5��CH3CH2OH��CH3OCH3��ֻд��1����1�֣�д2����3�֣�������������ͬ�����֣�
������������ˮ�����������ǣ���B�������ǡ���������ø�������·ֽ������Ҵ�����C���Ҵ����Ҵ������к����ǻ����ܷ���������������ȩ����D����ȩ����ȩ����ȩ���������������Ȼ�����E�����ᡣ�ױ��ڹ��յ������·���ȡ����Ӧ������±������±�������������Ƶ���Һ�з���ˮ�ⷴӦ���ɴ�����F�DZ��״������״������ᷢ��������Ӧ�������ᱽ����H��



 CH3CH2OH��NaCl
CH3CH2OH��NaCl
 RCH2OH��NaCl
RCH2OH��NaCl
 CH3CH2OH+NaCl
CH3CH2OH+NaCl