��Ŀ����
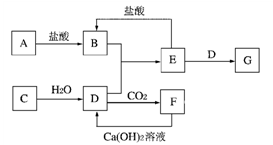
��8�֣���ͼ�и����ʾ�Ϊ��ѧ��ѧ�г��������ʣ�����֮������ͼת����ϵ������A��C��Ϊ�������ʣ�D����ɫ��Ӧ�ʻ�ɫ��C��ˮ��Ӧ��������D������������Ȼ����������壻E��һ��������������ܸ�NaOH��Ӧ���ܸ����ᷴӦ������Ӧ���������ɵ�ˮ��������������ȥ��
��ش��������⣺
��1��B��_________��E��_________�����ѧʽ��
��2��д��Eת��ΪG�����ӷ���ʽ ��
��3��д��C��ˮ��Ӧ�Ļ�ѧ����ʽ ��

��ش��������⣺
��1��B��_________��E��_________�����ѧʽ��
��2��д��Eת��ΪG�����ӷ���ʽ ��
��3��д��C��ˮ��Ӧ�Ļ�ѧ����ʽ ��

��

��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ
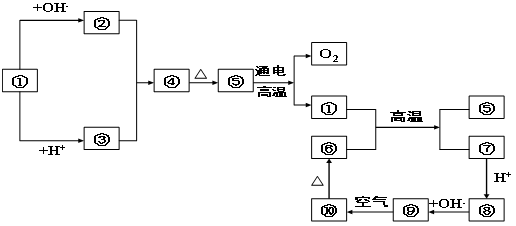
 ������װ��F��ƿ�У�þ������ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱڸ��ź�ɫ�������� C��D�������ɺ���ɫ���壻�� G��D��ȼ�տ��Բ���E��H2O���ཫB��H��ƿ�л�Ϻ����������ü����ӣ�ƿ�ڱڳ�����״Һ�β�����A���ش�
������װ��F��ƿ�У�þ������ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱڸ��ź�ɫ�������� C��D�������ɺ���ɫ���壻�� G��D��ȼ�տ��Բ���E��H2O���ཫB��H��ƿ�л�Ϻ����������ü����ӣ�ƿ�ڱڳ�����״Һ�β�����A���ش� ��
��
 ����Ӧ�ܵ����ӷ���ʽ ��
����Ӧ�ܵ����ӷ���ʽ �� ����A�����Ƿ����ķ����� ��
����A�����Ƿ����ķ����� �� ������C��
������C��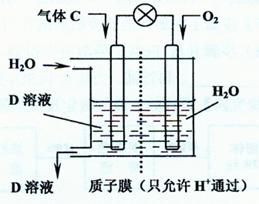
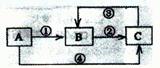
 ��ѧ��ѧ�еij������ʣ�����CΪO2��DΪC12��EΪFe���ʣ�����Ϊ��������Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��
��ѧ��ѧ�еij������ʣ�����CΪO2��DΪC12��EΪFe���ʣ�����Ϊ��������Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��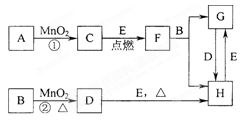
 �����ԡ����ԣ�ԭ�� ��
�����ԡ����ԣ�ԭ�� ��