��Ŀ����
��12�֣�����β��(�����ࡢCO��NO��SO2��)�dz�����Ҫ��ȾԴ֮һ�������İ취֮һ����������������װ��ת��������ʹNO��CO��Ӧ���ɿɲ��������̬ѭ���������壬��Ӧԭ����2NO(g)��2CO(g) N2(g)��2CO2(g)����298 K��100 kPa�£���H����113 kJ/mol����S����145 J/(mol��K)��
N2(g)��2CO2(g)����298 K��100 kPa�£���H����113 kJ/mol����S����145 J/(mol��K)��
��1��Ϊ����߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩΪ________��
| A���Ӵ���ͬʱ�����¶� | B���Ӵ���ͬʱ����ѹǿ |
| C�������¶�ͬʱ����N2 | D�������¶�ͬʱ����ѹǿ |
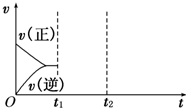
��3������ͼ�л�����ijʱ�������ı���ͼ��(������������)����_______ʱ���CO��ת�������

t1������NO��Ũ�� t2�������¶�
��4��ͨ�������жϸ������·�Ӧ�ܷ��Է����С�
(1)B��(2)����С
(3) ��t2�Ժ�
��t2�Ժ�
(4)��G����H��T��S
����113 kJ/mol��298 K��145��10��3 kJ/(mol��K)
����69.79 kJ/mol��0
�ʷ�Ӧ���Է�����
����

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
 ����β���������ࡢCO��NO��SO2�ȣ��dz�����Ҫ��ȾԴ֮һ�������İ취֮һ����������������װ��ת��������ʹNO��CO��Ӧ���ɿɲ��������̬ѭ���������壬��Ӧԭ����2NO��g��+2CO��g��?N2��g��+2CO2��g������298K��100kPa�£���H=-113kJ/mol����S=-145J/��mol?K����
����β���������ࡢCO��NO��SO2�ȣ��dz�����Ҫ��ȾԴ֮һ�������İ취֮һ����������������װ��ת��������ʹNO��CO��Ӧ���ɿɲ��������̬ѭ���������壬��Ӧԭ����2NO��g��+2CO��g��?N2��g��+2CO2��g������298K��100kPa�£���H=-113kJ/mol����S=-145J/��mol?K���� ����β���������ࡢCO��NO��SO2�ȣ��dz�����Ҫ��ȾԴ֮һ�������İ취֮һ����������������װ��ת��������ʹNO��CO��Ӧ���ɿɲ��������̬ѭ���������壬��Ӧԭ����2NO��g��+2CO��g���TN2��g��+2CO2��g������298K��100kPa�£���H=-113kJ/mol����S=-145J/��mol?K����
����β���������ࡢCO��NO��SO2�ȣ��dz�����Ҫ��ȾԴ֮һ�������İ취֮һ����������������װ��ת��������ʹNO��CO��Ӧ���ɿɲ��������̬ѭ���������壬��Ӧԭ����2NO��g��+2CO��g���TN2��g��+2CO2��g������298K��100kPa�£���H=-113kJ/mol����S=-145J/��mol?K����