��Ŀ����
��07�꽭�վ�����10�֣���ҵ����������Ϊԭ����������������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ������Ṥҵ���ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ���밴Ҫ��ش��������⣺
(1)��β��ͨ�백ˮ�У��ܷ��������Ӧ��д�����п��ܷ���������������ԭ��Ӧ�Ļ�ѧ����ʽ�� ��
(2)��β���백ˮ��Ӧ���õ��ĸ�Ũ����Һ�У���һ���������백ˮ��̼����泥���ʱ��Һ���¶Ȼ����н��ͣ����������塣
�ٵ�����Һ�¶Ƚ��͵�ԭ������� ��
�������ľ����������ֽ��ҵ��Ҳ��������������ӰҺ����������֪�ýᾧˮ�������Է�������Ϊ134�����仯ѧʽΪ ��
��������������Ҫ����Һ�м��������ĶԱ����ӻ�Ա����������ʣ���Ŀ����
��3�������ڲⶨ����β����SO2�������� ��������ĸ��
A.NaOH��Һ����̪��Һ B.KMnO4��Һ��ϡH2SO4
C.��ˮ��������Һ D.��ˮ����̪��Һ
�𰸣�

���������⿼���˹�ҵ�������ԭ���������������⡣���Ƹ���ʵ��ֻ�漰��һЩ������ԭԭ���Լ���Ӧ�������仯�����֪ʶ����
ϸ�����������ؼ������ܽ���ô���Ĵ𰸡�+4����Ԫ���ܱ����������õ�+6�ۡ�

��07�꽭�վ�����8�֣����ȩ��һ��ʳ���㾫�����㷺�������ࡢϴ�Ӽ����ǹ��Լ���ζƷ�С�
��ҵ�Ͽ�ͨ�����з�Ӧ�Ʊ���
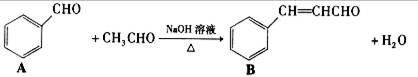 |
��1�����Ʋ�B�����Ͽ��ܷ�����Ӧ�����ͣ�_____ ���������֣�
��2����д����������ȩ�����������·�Ӧ�Ļ�ѧ����ʽ��_____ ��
��3����д��ͬʱ���������������µ�����ͬ���칹��Ľṹ��ʽ��______ ��
���ٷ����в����ʻ����ǻ� ���DZ��ĶԶ�ȡ���� �۳������⣬����������״�ṹ����
��07�꽭�վ���(10��)������ҵ�ǹ��ҹ�ҵ�Ļ�����2006���ҹ��ֲָ���ͻ��4�ڶ֣���������λ��ij��ѧ���ʵ���С�����ü��ڶԵ��ظ����������˵��У��Դӿ�ʯ��ʼ�����������Ĺ�����������ȫ��ĸ�����ʶ�����������ʵ���С�����Ȥ��������м��㣺
(1)��6��62 g����ʯ��ƷͶ��������������(��ַ�Ӧ)�����ˣ�Ȼ������Һ�мӹ�����NaOH ��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����յ�4.80g Fe2O3�����Ը�����ʯΪԭ����������������������Ԫ����ʧ4��������ÿ����1.00t����(����96��)��������Ҫ��������ʯ���ٶ�? (������λС��)
(2)ȡij������ĩ28.12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״��)��
�� ����˸�������������̼�����ʵ���֮�ȡ�
����ȡ���ݲ�ͬ�����ĸ�����ĩ �ֱ�ӵ�100mL��ͨŨ�ȵ�H2SO4��Һ�У���ַ� Ӧ�� ��õ�ʵ���������±���ʾ��
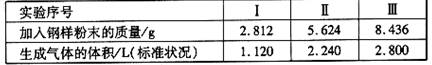 |
����������Һ�����ʵ���Ũ�ȡ�
������ʵ����м�������mg������ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ����?(�ú�m�Ĵ���ʽ��ʾ)
