��Ŀ����
��6�֣���������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���
��1����250C��101KPaʱ��3.2�˼״���CH30H����ȫȼ������CO2��Һ̬ˮʱ����72.576kJ�����ܱ�ʾ�״�ȼ�յ��Ȼ�ѧ����ʽΪ___________________��
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ2 L���ܱ������У�����2mol CO2��6mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49kJ/mol�����CO2��H2O (g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ��H����49kJ/mol�����CO2��H2O (g)��Ũ����ʱ��仯��ͼ��ʾ��
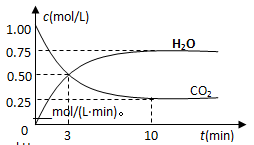
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��__________mol/(L��min)��
�����д�ʩ����ʹn(H2O)��n(CO2)�������________��
A�������¶� B���ٳ���3mol H2
C����CH3OH (g)����ϵ�з��� D������He(g)��ʹ��ϵѹǿ����
��1����250C��101KPaʱ��3.2�˼״���CH30H����ȫȼ������CO2��Һ̬ˮʱ����72.576kJ�����ܱ�ʾ�״�ȼ�յ��Ȼ�ѧ����ʽΪ___________________��
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ2 L���ܱ������У�����2mol CO2��6mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)
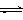 CH3OH(g)��H2O(g) ��H����49kJ/mol�����CO2��H2O (g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ��H����49kJ/mol�����CO2��H2O (g)��Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��__________mol/(L��min)��
�����д�ʩ����ʹn(H2O)��n(CO2)�������________��
A�������¶� B���ٳ���3mol H2
C����CH3OH (g)����ϵ�з��� D������He(g)��ʹ��ϵѹǿ����
��6�֣���1��CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=-725.76kJ��mol-1
��2����0.225 mol/(L��min)����B C ��ÿ��2�֣�
��2����0.225 mol/(L��min)����B C ��ÿ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2Z��ӦӰ���ʾ��ͼ��ͼ���������ʾƽ����������Z���������������������ȷ���ǣ� ��
2Z��ӦӰ���ʾ��ͼ��ͼ���������ʾƽ����������Z���������������������ȷ���ǣ� ��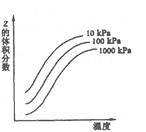
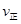 ����
����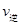 ��С��ƽ�������ƶ�
��С��ƽ�������ƶ� p C��g�����ﵽƽ��ʱ�������¶Ȳ��䣬�������ݻ�ѹ����ԭ����һ�룬���ﵽ�µ�ƽ��ʱ��B��Ũ��Ϊԭ����2.5��������˵����ȷ���ǣ� ��
p C��g�����ﵽƽ��ʱ�������¶Ȳ��䣬�������ݻ�ѹ����ԭ����һ�룬���ﵽ�µ�ƽ��ʱ��B��Ũ��Ϊԭ����2.5��������˵����ȷ���ǣ� ��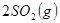
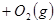

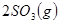 ��������ʼ�����ﵽ��ƽ�⣬��ʱ
��������ʼ�����ﵽ��ƽ�⣬��ʱ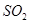 ��
��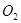 �����ʵ���֮��Ϊ2��1������ȡij�ִ�ʩ������
�����ʵ���֮��Ϊ2��1������ȡij�ִ�ʩ������ ��Ũ�ȿ϶�����
��Ũ�ȿ϶����� N2O4ƽ�⣬ƽ��ʱNO2��N2O4���ʵ���Ũ��֮��Ϊ�����������������£��ֱ��ٳ���NO2���ٳ���N2O4��ƽ�������𦵵ı仯��ȷ���ǣ��� ��
N2O4ƽ�⣬ƽ��ʱNO2��N2O4���ʵ���Ũ��֮��Ϊ�����������������£��ֱ��ٳ���NO2���ٳ���N2O4��ƽ�������𦵵ı仯��ȷ���ǣ��� �� N2O4��g����H=-57.2kJ��mol-1
N2O4��g����H=-57.2kJ��mol-1
 ���ڡ�����С�����ڡ���
���ڡ�����С�����ڡ��� ���䣬���м��ܼӿ�����Ӧ�����������NO2ת���ʵĴ�ʩ��
���䣬���м��ܼӿ�����Ӧ�����������NO2ת���ʵĴ�ʩ�� 2Z(g)��
2Z(g)��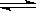 XC(g)������ͼ����ʾ��ϵ���ɴ��ƶ϶�ͼ������ȷ˵���ǣ� ��
XC(g)������ͼ����ʾ��ϵ���ɴ��ƶ϶�ͼ������ȷ˵���ǣ� ��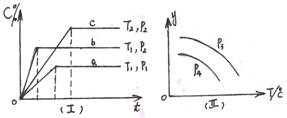

 Z(g)����60s�ﵽƽ������0��3mol��Z������˵����ȷ����( )
Z(g)����60s�ﵽƽ������0��3mol��Z������˵����ȷ����( )