��Ŀ����
��̼���⡢������Ԫ����ɵ�ij�л���X����Է�������Ϊ136������̼����Ԫ�ص���������֮��Ϊ76.5%����֪X�к��м��������ϵ�һ�ȴ��������֣�X���ȵ�����KMnO4��Һ������ת��ΪY��1mol Y������NaHCO3��Һ��Ӧ���ɱ�״̬��44.8L CO2���壮
�ݴ��������Ҫ��
��1��X�к��������ŵ�����Ϊ______��1mol Y������______mol Na��Ӧ��
��2����֪A��B��C��D����X��Ϊͬ���칹�壬�Ҿ�����һ��ȡ�����ķ����廯�������B���Է���������Ӧ�����ת����ϵ����ͼ��
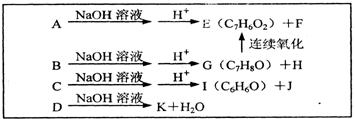
��A�Ľṹ��ʽΪ______��A��K�ĸ��л����У����������______������ĸ����
��C��������NaOH��Һ����ʱ������Ӧ�Ļ�ѧ����ʽΪ��______���÷�Ӧ���漰�����л���Ӧ����Ϊ______��
�����ڷ����廯�����E��ͬ���칹����______�֣�����E����
��������G��Y��һ�������·�����Ӧ�Ļ�ѧ����ʽΪ��______��
�ݴ��������Ҫ��
��1��X�к��������ŵ�����Ϊ______��1mol Y������______mol Na��Ӧ��
��2����֪A��B��C��D����X��Ϊͬ���칹�壬�Ҿ�����һ��ȡ�����ķ����廯�������B���Է���������Ӧ�����ת����ϵ����ͼ��

��A�Ľṹ��ʽΪ______��A��K�ĸ��л����У����������______������ĸ����
��C��������NaOH��Һ����ʱ������Ӧ�Ļ�ѧ����ʽΪ��______���÷�Ӧ���漰�����л���Ӧ����Ϊ______��
�����ڷ����廯�����E��ͬ���칹����______�֣�����E����
��������G��Y��һ�������·�����Ӧ�Ļ�ѧ����ʽΪ��______��
��1��̼����Ԫ�ص���������֮��Ϊ76.5%����OԪ�ص���������Ϊ1-76.5%=23.5%���������Oԭ�ӵĸ���Ϊ
=2����Ϊ�ṹ�к��м��������ϵ�һ�ȴ��������֣�˵����������2����ͬ��ȡ��������λ�ڶ�λ�����ȵ�����KMnO4��Һ������ת��ΪY��1mol Y������NaHCO3��Һ��Ӧ���ɱ�״̬��44.8L CO2���壬˵��Y�к���2��-COOH����XӦ����1������1���Ȼ����ṹ��ʽΪ
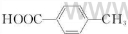
���ȵ�����KMnO4��Һ������ת��ΪY��Ϊ

��1mol

����2molNaOH��Ӧ���ʴ�Ϊ���Ȼ��� 2mol��
��2����ΪA��B��C��D����X��Ϊͬ���칹�壬�Ҿ�����һ��ȡ�����ķ����廯�������ܵĽṹ��
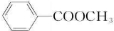
��

��
��
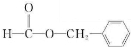
�ȣ���Ͽ�ͼG���������õ�E���ƶ�GΪ����EΪ�ᣬ��AΪ
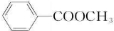
��BΪ
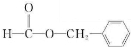
������-CHO�����Է���������Ӧ��CΪ

��DΪ

��
�������Ϸ�����֪AΪ
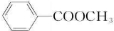
����A��B��C��D�����ʿ�֪���������Ե���D��E�������ᣩ��H�����ᣩ��J�����ᣩ���ʴ�Ϊ��
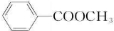
��D��E��H��J��
��CΪ

����������NaOH��Һ����ʱ������Ӧ�Ļ�ѧ����ʽΪ

��
�ʴ�Ϊ��

��ȡ����ˮ�⣻
��EΪ�����ᣬ��Ӧ�����ڷ����廯�����ͬ���칹����

��

���ڡ��䡢�ԣ���4�֣��ʴ�Ϊ��4��
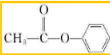
��GΪ���״���YΪ

�����߿ɷ���������Ӧ������ʽΪ

��
�ʴ�Ϊ��

��
| 136��23.5% |
| 16 |

���ȵ�����KMnO4��Һ������ת��ΪY��Ϊ

��1mol

����2molNaOH��Ӧ���ʴ�Ϊ���Ȼ��� 2mol��
��2����ΪA��B��C��D����X��Ϊͬ���칹�壬�Ҿ�����һ��ȡ�����ķ����廯�������ܵĽṹ��
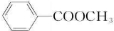
��

��

��
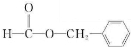
�ȣ���Ͽ�ͼG���������õ�E���ƶ�GΪ����EΪ�ᣬ��AΪ
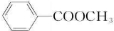
��BΪ
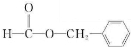
������-CHO�����Է���������Ӧ��CΪ

��DΪ

��
�������Ϸ�����֪AΪ
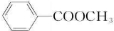
����A��B��C��D�����ʿ�֪���������Ե���D��E�������ᣩ��H�����ᣩ��J�����ᣩ���ʴ�Ϊ��
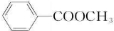
��D��E��H��J��
��CΪ

����������NaOH��Һ����ʱ������Ӧ�Ļ�ѧ����ʽΪ

��
�ʴ�Ϊ��

��ȡ����ˮ�⣻
��EΪ�����ᣬ��Ӧ�����ڷ����廯�����ͬ���칹����

��

���ڡ��䡢�ԣ���4�֣��ʴ�Ϊ��4��
��GΪ���״���YΪ

�����߿ɷ���������Ӧ������ʽΪ

��
�ʴ�Ϊ��

��

��ϰ��ϵ�д�
�����Ŀ
 +2H2O
+2H2O