��Ŀ����
��1��(3��)���λ�����Ⱦ��������̬�����ѳ�Ϊȫ��Ĺ�ʶ��
�ٿ�����������ĸ���ָ����Է�ӳ�����ؿ��������������������������ҹ���������������� (����ĸ)��
a��CO2 b��N2 c��NO2
������Ӧ�����ռ������¡���ɫ��Ⱦ������������Ӧ���������� (����ĸ)��־������Ͳ�ڡ�

�۹�ҵ��ˮ�账����������ŷš����з�ˮ�����ķ����������� (����ĸ)��
a�����кͷ���ȥ��ˮ�е���
b���û�������ȥ��ˮ�е��ؽ�������
c����������ȥ��ˮ�е�������
��2����5�֣�������ָpH___________�Ľ�ˮ������ȼ�պ���ú������������ˮ���䵽���棬pH��ʱ��䳤��������С�����û�ѧ����ʽ������ԭ��


��3����5�֣�����β����һ��������һ����̼��ת���� ��ѧ����ʽΪ ���������˸����ݶ��ṩ��ֱ��ˮ����ǰ�ڴ����γ�������������ClO2����ˮ�������е�Al2(SO4)3��ˮ�����ɴ������Al(OH)3���壬������Ӧ�����ӷ���ʽ�� ��ClO2�������� ��
��ѧ����ʽΪ ���������˸����ݶ��ṩ��ֱ��ˮ����ǰ�ڴ����γ�������������ClO2����ˮ�������е�Al2(SO4)3��ˮ�����ɴ������Al(OH)3���壬������Ӧ�����ӷ���ʽ�� ��ClO2�������� ��
�ٿ�����������ĸ���ָ����Է�ӳ�����ؿ��������������������������ҹ���������������� (����ĸ)��
a��CO2 b��N2 c��NO2
������Ӧ�����ռ������¡���ɫ��Ⱦ������������Ӧ���������� (����ĸ)��־������Ͳ�ڡ�

�۹�ҵ��ˮ�账����������ŷš����з�ˮ�����ķ����������� (����ĸ)��
a�����кͷ���ȥ��ˮ�е���
b���û�������ȥ��ˮ�е��ؽ�������
c����������ȥ��ˮ�е�������
��2����5�֣�������ָpH___________�Ľ�ˮ������ȼ�պ���ú������������ˮ���䵽���棬pH��ʱ��䳤��������С�����û�ѧ����ʽ������ԭ��


��3����5�֣�����β����һ��������һ����̼��ת����
 ��ѧ����ʽΪ ���������˸����ݶ��ṩ��ֱ��ˮ����ǰ�ڴ����γ�������������ClO2����ˮ�������е�Al2(SO4)3��ˮ�����ɴ������Al(OH)3���壬������Ӧ�����ӷ���ʽ�� ��ClO2�������� ��
��ѧ����ʽΪ ���������˸����ݶ��ṩ��ֱ��ˮ����ǰ�ڴ����γ�������������ClO2����ˮ�������е�Al2(SO4)3��ˮ�����ɴ������Al(OH)3���壬������Ӧ�����ӷ���ʽ�� ��ClO2�������� ����1��(3��) ��c ��a ��a (ÿ ��1�֣���3��)
��1�֣���3��)
��2����5�֣� ��5.6 SO2+H2O=H2SO3 2H2SO3+O2=2H2SO4������ʽ2�֣�
 ��3�� 2NO��2CO
��3�� 2NO��2CO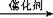 N2��2CO2��2�֣�Al3++3H2O Al(OH)3(����)+3H+ (2��)
N2��2CO2��2�֣�Al3++3H2O Al(OH)3(����)+3H+ (2��)
ɱ������ (�������ķ�������ÿ��1�֣���ͬ)
 ��1�֣���3��)
��1�֣���3��)��2����5�֣� ��5.6 SO2+H2O=H2SO3 2H2SO3+O2=2H2SO4������ʽ2�֣�
 ��3�� 2NO��2CO
��3�� 2NO��2CO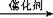 N2��2CO2��2�֣�Al3++3H2O Al(OH)3(����)+3H+ (2��)
N2��2CO2��2�֣�Al3++3H2O Al(OH)3(����)+3H+ (2��) ɱ������ (�������ķ�������ÿ��1�֣���ͬ)
��

��ϰ��ϵ�д�
�����Ŀ
 �γɵ�
�γɵ� ��ϴ��ʱ��ˮ��Ҫ��ϸߣ�����ˮ����ˮ�ж�����ʹ��
��ϴ��ʱ��ˮ��Ҫ��ϸߣ�����ˮ����ˮ�ж�����ʹ��