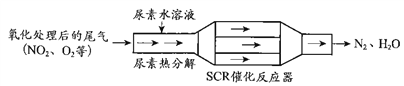
��Ŀ����
����Ŀ���ҹ�����ר�Һ�°Ľ����������������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�����������ͼ1:

��1������йط�Ӧ�Ļ�ѧ����ʽ
�ٳ����أ�NH3+CO2+H2O��NaCl=NaHCO3��NH4Cl
������¯��_______________��
��2�������Ƽ���ŵ�����У�����ȷ����_______��
A������ԭ��Ϊ��ʳ�Ρ�NH3��CO2
B���������Ȼ�刺�������
C�����������п�ѭ�����õ�����ֻ��CO2
D��ԭ�������ʸ�
ijʵ��С�飬��������װ��ͼ2ģ���������Ƽ���ĵ�һ����Ӧ��

��3������װ���нӿ�����˳��Ϊ______��
A.a��c��b��f��e��d B.a��d��b��f��e��c
C.b��d��a��e��f��c D.b��c��a��f��e��d
��4��D��Ӧѡ�õ�Һ��Ϊ______________��Ϊ�ⶨ��Ʒ����ijɷֺͺ�����������ʵ�飬�����Ʒ������ֻ��NaCI��NaHCO3���ʡ�
��5�������Ʒ�������Ƿ���NaCl����ȡ������������ˮ���ٵμ�___________�Լ���
��6���ζ����ⶨ�����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷWg��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ������cmol/L��HCI��Һ�ζ�����Һ�ɺ�ɫ��Ϊ��ɫ��ָʾCO32-+H+=HCO3-��Ӧ���յ㣩������HCI ��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻ�ɫ��Ϊ��ɫ������HCI��Һ�����ΪV2mL������Ʒ��NaHCO3��������Ϊ____________��
���𰸡� 2NaHCO3===Na2CO3+CO2+H2O C D ����NaHCO3��Һ ϡHNO3��AgNO3��Һ ![]()
��������������Ҫ����̼�����Ƶ��ƵĻ���������ʡ�
��1���йط�Ӧ�Ļ�ѧ����ʽ��
�ٳ����أ�NH3+CO2+H2O��NaCl=NaHCO3��NH4Cl
������¯��2NaHCO3![]() Na2CO3+H2O+CO2����
Na2CO3+H2O+CO2����
��2�������Ƽ�����������п�ѭ�����õ�������CO2��NaCl����ѡC��
��3��װ��B����NH3��װ��A����CO2�����к�������ӷ�����HCl����Ҫ����װ��D��ȥװ��CO2�е�HCl���ʣ�NH3��CO2��װ��C��NaCl������Ӧ������������ˮ����Ҫ��ֹ���������Ը������ڵ�����˳��Ϊ����װ���нӿ�����˳��Ϊb��c��a��f��e��d����ѡD��
��4��D��Ӧѡ�õ�Һ��Ϊ����NaHCO3��Һ��
��5�������Ʒ�������Ƿ���NaCl����ȡ������������ˮ���ٵμ�ϡHNO3��AgNO3��Һ�Լ���
��6��Na2CO3+HCl![]() NaHCO3+NaCl��������V1mL��NaHCO3+HCl
NaHCO3+NaCl��������V1mL��NaHCO3+HCl![]() NaCl+H2O+CO2����������V2mL������ԭ̼�����Ƶ����ʵ���Ϊc(V2-V1)����˴�����Ʒ��NaHCO3��������Ϊ
NaCl+H2O+CO2����������V2mL������ԭ̼�����Ƶ����ʵ���Ϊc(V2-V1)����˴�����Ʒ��NaHCO3��������Ϊ![]() ��
��