��Ŀ����
����Ϊ��ѧ��ѧ�������ʣ������ʼ�������ת����ϵ��
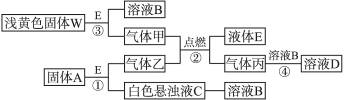
(1)WΪ_____________________��
(2)����A��һ����Ҫ�Ĺ�ҵԭ�ϣ�1 mol��������ȫȼ��ʱ����1 mol E��2 mol�������ҵķ���ʽΪ______________________��
(3)C��Һ�е����ʺͱ������ʵ���֮��1��2��Ӧʱ�������ʶ��������ӷ���ʽΪ________________________��
(4)����ͼ���й�����ʵ��C��B��ת�䣬��������________________(�ѧʽ)��
(5)������ǡ����ȫ��Ӧ����A��W�����ʵ���֮��Ϊ______________________��
(6)����W�붡�����ʵ���1 mol��1 mol��ϲ����ܱ������г�ַ�Ӧ���������ɹ��弰���ʵ���Ϊ______________________________��
(1)Na2O2 (2)C2H2 (3)CO2+OH-====![]() (4)Na2CO3
(4)Na2CO3
(5)1��2 (6)Na2CO3��CaCO3��1 mol
����:
���ʵ���ɫ�����ֵ㡣dz��ɫ����WΪNa2O2����ϵNa2O2���й����ʵķ�Ӧ��ϸ�ͼʾ������֪Һ��EΪH2O������BΪNaOH��Һ�������ΪO2��������(2)��֪AΪCaC2��������ΪC2H2����ɫ����ҺCΪCa(OH)2�������ΪCO2����Ϥ���ʵ���֮���ת����ϵ���Լ�����֪�й����ݡ�

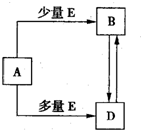 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ���� ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����

