��Ŀ����
����������ӷ���ʽ��д��ȷ���� (����)��
| A��NaClO��Һ��FeCl2��Һ��ϣ�6Fe2����3ClO����3H2O=2Fe(OH)3����3Cl����4Fe3�� |
| B����ʳ���������е�̼��ƣ�CaCO3��2H��=Ca2����CO2����H2O |
| C��FeCl2������Һ���ڿ����б��ʣ�2Fe2����4H����O2=2Fe3����2H2O |
D�����MgCl2ˮ��Һ�����ӷ���ʽ��2Cl����2H2O H2����Cl2����2OH�� H2����Cl2����2OH�� |
A
ѡ��A��ClO����Fe2������ΪFe3����A����ȷ��ѡ��B��ʳ����Ч�ɷ�Ϊ���ᣬ����Ӧ��д����ʽ����ȷ�����ӷ���ʽΪCaCO3��2CH3COOH=Ca2����2CH3COO����CO2����H2O��ѡ��C�е�ʧ���Ӻ͵�ɲ��غ㣬��ȷ�����ӷ���ʽΪ4Fe2����4H����O2=4Fe3����2H2O��ѡ��D�е���Ȼ�þˮ��Һ���ɵ�OH����Mg2����Ӧ����������þ��������ȷ�����ӷ���ʽΪMg2����2Cl����2H2O Mg(OH)2����H2����Cl2����
Mg(OH)2����H2����Cl2����
 Mg(OH)2����H2����Cl2����
Mg(OH)2����H2����Cl2����
��ϰ��ϵ�д�
�����Ŀ


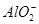 ��4
��4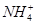

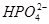 ��H2O
��H2O
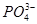 ��H3O��
��H3O��
 HCO3¯+OH¯
HCO3¯+OH¯ 2CrO42��+2H+
2CrO42��+2H+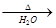 CH3CH2OH +Br-
CH3CH2OH +Br-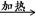 2C6H5OH + CO32-
2C6H5OH + CO32-