��Ŀ����
ϡHNO3��п��Ӧ�Ļ�ѧ����ʽ���£�4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O
��1����˫���ű���������ѧ����ʽ�е���ת�Ƶķ������Ŀ______��
��2���÷�Ӧ�л�ԭ����______����ԭ������______��ÿ2mol Zn�μӷ�Ӧ��ת�Ƶĵ���______����
��3��������Ӧ�У�HNO3û��ȫ���μ�������ԭ��Ӧ��û�вμ�������ԭ��Ӧ������������������ʵ���֮����______��
��1����˫���ű���������ѧ����ʽ�е���ת�Ƶķ������Ŀ______��
��2���÷�Ӧ�л�ԭ����______����ԭ������______��ÿ2mol Zn�μӷ�Ӧ��ת�Ƶĵ���______����
��3��������Ӧ�У�HNO3û��ȫ���μ�������ԭ��Ӧ��û�вμ�������ԭ��Ӧ������������������ʵ���֮����______��
��1���ڷ�Ӧ4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O�У�ZnԪ�ػ��ϼ����ߣ���0������Ϊ+2�ۣ�ʧȥ���ӣ�NԪ�ػ��ϼ۽��ͣ���+5�۽���Ϊ+1�ۣ��õ����ӣ�����ת�Ƶķ������Ŀ�ɱ�ʾΪ
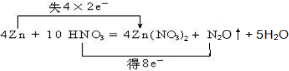
��
�ʴ�Ϊ��

��
��2���ɻ��ϼ۱仯��֪��ԭ��ΪZn��N2OΪ��ԭ���ÿ2mol Zn�μӷ�Ӧ��ת�Ƶĵ���Ϊ4NA��
�ʴ�Ϊ��Zn��N2O��4NA��
��3���ɻ��ϼ۱仯��֪10mol����μӷ�Ӧ��ֻ��2mol����ԭ����û�вμ�������ԭ��Ӧ������������������ʵ���֮����4��5���ʴ�Ϊ��4��5��

��
�ʴ�Ϊ��

��
��2���ɻ��ϼ۱仯��֪��ԭ��ΪZn��N2OΪ��ԭ���ÿ2mol Zn�μӷ�Ӧ��ת�Ƶĵ���Ϊ4NA��
�ʴ�Ϊ��Zn��N2O��4NA��
��3���ɻ��ϼ۱仯��֪10mol����μӷ�Ӧ��ֻ��2mol����ԭ����û�вμ�������ԭ��Ӧ������������������ʵ���֮����4��5���ʴ�Ϊ��4��5��

��ϰ��ϵ�д�
�����Ŀ
