��Ŀ����
�±���Ԫ�����ڱ���һ���֣���Ա��Т١����е�Ԫ�أ��ش��������⣺
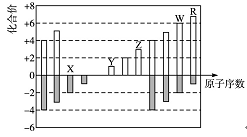
��1���ڱ���a��b��c��d�ĸ������У�ȫ���ǽ���Ԫ�ص�����Ϊ ��
��2���ڢ١���Ԫ���У�ԭ�Ӱ뾶������ �� ����Ԫ�����ƣ����ٺ͢���ɵĻ�����ĵ���ʽΪ ��������ӽṹʾ��ͼΪ ��
��3��Ԫ�ص�����������Ӧ��ˮ������������ǿ���� ���ѧʽ����ͬ����������ǿ������ �������Ե��� ��
��4����͢��У��ǽ����Խ�ǿ���� ����Ԫ�ط��ţ�������һ����ѧ��Ӧ˵������ʵ��д���÷�Ӧ�����ӷ���ʽ�� ��
| �� | | | |||||||||||||||||||||||
| | | | | �� | �� | �� | �� | | |||||||||||||||||
| �� | �� | �� | | | | �� | | ||||||||||||||||||
| | | | | |
| | | | | | | |
| |
| | ||||||||
| | | | | | | | | | | | | | | | | | | ||||||||
| | | | | | | | | | | | | | | | | | | ||||||||
| | | | | | | | | | | ||||||||||||||||
��2���ڢ١���Ԫ���У�ԭ�Ӱ뾶������ �� ����Ԫ�����ƣ����ٺ͢���ɵĻ�����ĵ���ʽΪ ��������ӽṹʾ��ͼΪ ��
��3��Ԫ�ص�����������Ӧ��ˮ������������ǿ���� ���ѧʽ����ͬ����������ǿ������ �������Ե��� ��
��4����͢��У��ǽ����Խ�ǿ���� ����Ԫ�ط��ţ�������һ����ѧ��Ӧ˵������ʵ��д���÷�Ӧ�����ӷ���ʽ�� ��
��ÿ��1�֣���9�֣�
��1��b
��2���ƣ�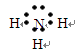 ��
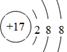
��
��3��HClO4��NaOH��Al(OH)3
��4��Cl�� Cl2+2Br-��2Cl-+Br2
��1��b
��2���ƣ�
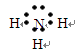 ��
��
��3��HClO4��NaOH��Al(OH)3
��4��Cl�� Cl2+2Br-��2Cl-+Br2
�����������1��b���������ǹ��Ƚ�������ȫ���ǽ���Ԫ�ص�����b��
��2��ͬ���ڣ��������ң�ԭ�Ӱ뾶��С��ͬ���壬�������£�ԭ�Ӱ뾶������ԭ�Ӱ뾶�����Ǣޣ����ơ��ٺ͢���ɵĻ�������NH3������ʽΪ
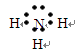 ������Cl�������ӽṹʾ��ͼΪ
������Cl�������ӽṹʾ��ͼΪ ��
����3��ͬ���ڣ��������ң��������������ǽ���������ǿ��ͬ���壬�������£�����������ǿ���ǽ�����������Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ������������ǿ����ΪHClO4��Ԫ�صĽ�����Խǿ��������������Ӧ��ˮ���������ǿ�ģ���ΪNaOH�������Ե���Al(OH)3��
��4��ͬ���ڣ��������ң��������������ǽ���������ǿ��ͬ���壬�������£�����������ǿ���ǽ������������ʷǽ����Ԣ���ڢ⣬�ǽ����Խ�ǿ����Cl��֤���ǽ�����ǿ���Ļ�ѧ��Ӧ�����ӷ���ʽΪCl2+2Br-��2Cl-+Br2��
���������⿼��ѧ��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�֪ʶ����һ���ۺ��⣬�ѶȽϴ�

��ϰ��ϵ�д�
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ