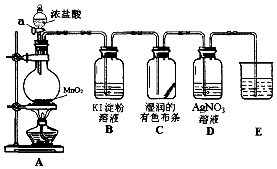
��Ŀ����
2����ú��ʯ�͡���Ȼ������������в����ĺ�H2S�����������л������ÿ���˹����һ�ָĽ����¹����������£�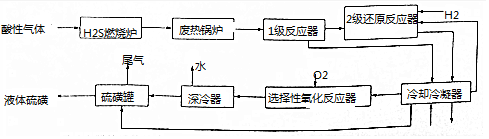
��֪��2����ԭ��Ӧ����Ŀ���ǽ�������SO2��������ȫ��ת��ΪH2S��
�ش��������⣺
��1����ȼ��¯�н�Լ1/3��H2Sȼ������SO2�Ļ�ѧ����ʽΪ2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O�������з��ȹ�¯����Ҫ�����ǻ�������ȼ�ղ����IJ���������
��2���ڢ�Ӧ�������ɵ�SO2��ʣ���H2S����S�Ļ�ѧ����ʽΪ2H2S+SO2=3S��+2H2O��ȼ��¯��ֻȼ�յ�1/3H2S��Ŀ���Ǽ���H2��������
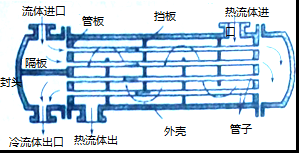
��3��һ����ȴ���ṹ��ͼ�����ֲ�ͬ�����������ԭ�������ŵ����Ƚ���Ч�ʸߣ�
��4��ѡ����������Ӧ����ͨ��������������Ӧ�Ļ�ѧ����ʽΪ2H2S+O2$\frac{\underline{\;һ������\;}}{\;}$2S��+2H2O��
��5����ƹ�β����ʯ���鴦�����ŷţ�ʯ��������������ռ�������H2S��SO2����ֹ��Ⱦ������
��6�����к�H2S�������Ϊ80%����������22.4m3����״���������ջ��յ��Ĵ���Ϊ96%�ĵ�����25.0Kg������Ļ�����Ϊ93.75%��
���� ��1����ȼ��¯�н�Լ1/3��H2Sȼ������SO2����Ӧ��Ϊ���⣬����Ϊ���������ˮ�����÷��ȹ�¯�������������Ȼ磻
��2������Ͷ�������Ӧ�������ˮ��ȼ��¯��ֻȼ�յ�1/3H2S��Ŀ���Ǽ���H2��������
��3����ȴ�����ֲ�ͬ�����������ԭ����������Ƚ���Ч�ʣ�
��4��ѡ����������Ӧ����ͨ����������������������ַ�Ӧ�������ˮ��
��5��ʯ����ɷ�Ϊ�������ƣ������ն����������⣬���ٶԿ�������Ⱦ��
��6��������ԭ���غ㣬�������������������������ݻ�����=$\frac{ʵ�ʲ���}{���۲���}$��100%���㣮
��� �⣺��1�������������Ӧ���ɶ��������ˮ����Ӧ����ʽΪ��2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O�������з��ȹ�¯��������ȼ�ղ����IJ����������ɳ�����÷�Ӧ�ų������������������ɱ���
�ʴ�Ϊ��2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O����������ȼ�ղ����IJ���������
��2������Ͷ������������̬���з�Ӧ��H2S��SԪ�صĻ��ϼ����ߣ�����������Ӧ�������ˮ����Ӧ�ķ���ʽΪ��2H2S+SO2=3S��+2H2O��ȼ��¯��ֻȼ�յ�1/3H2S��Ŀ���Ǽ���H2��������
�ʴ�Ϊ��2H2S+SO2=3S��+2H2O������H2��������
��3�����Ƚ����У���ĺ��ȵ����ֲ�ͬ���壬���������ķ������������Ľ�������ʹ���������ӷ֣�������Ƚ���Ч�ʣ�
�ʴ�Ϊ���Ƚ���Ч�ʸߣ�
��4������Ͳ�������������Ӧ�������ˮ����Ӧ����ʽΪ��2H2S+O2$\frac{\underline{\;һ������\;}}{\;}$2S��+2H2O��
�ʴ�Ϊ��2H2S+O2$\frac{\underline{\;һ������\;}}{\;}$2S��+2H2O��
��5����ƹ�β��������������H2S��SO2��ʯ����ɷ�Ϊ�������ƣ������ն����������⣬��ʯ���鴦�����ŷţ����ռ�������H2S��SO2����ֹ��Ⱦ������
�ʴ�Ϊ�����ռ�������H2S��SO2����ֹ��Ⱦ������
��6����H2S�������Ϊ80%����������22.4m3����״�����������۲���Ϊ$\frac{22.4��1{0}^{3}L��80%}{22.4L/mol}��32g/mol$=25600g�����ջ��յ��Ĵ���Ϊ96%�ĵ�����25.0Kg��������=$\frac{ʵ�ʲ���}{���۲���}$��100%=$\frac{25600g}{25.0Kg��96%}$=93.75%��
�ʴ�Ϊ��93.75%��
���� ���⿼�麬H2S�����������л������գ��������仯����������ǽ��ؼ�����6������ע��ԭ���غ��Ӧ�ã���Ŀ�Ѷ��еȣ�

| A�� | N2��O2��Cl2�����мȺ��ЦҼ����ֺ��Цм� | |
| B�� | PCl3�����У�����ԭ�Ӳ�ȡSP3�ӻ���ʽ | |
| C�� | CS2��H2O��C2H2����ֱ���ͷ��� | |
| D�� | CH4��CCl4��C6H6����ֻ���м��Լ��ķǼ��Է��� |
| A�� | ������FeCl3��Һ��εμӵ�NaOH��Һ�У������Ƶ�Fe��OH��3���� | |
| B�� | FeCl3��Һ�ǵ����Եģ�Fe��OH��3�����Ǵ�����ɵ� | |
| C�� | �����ö������������FeCl3��Һ��Fe��OH��3���� | |
| D�� | ��500mL 2mol/L FeCl3��Һ�Ƴɽ��壬��������ĿΪNA |
| A�� | 11�� | B�� | 10�� | C�� | 9�� | D�� | 8�� |
| A�� | SO2���л�ԭ�ԣ��ʿ���Ư�� | |
| B�� | Na�Ľ�����Ա�Mgǿ���ʿ���Na��MgCl2��Һ��Ӧ��Mg | |
| C�� | Ũ�����е�HNO3�����ֽ⣬����ʱ��ʵ���ҿ�����Ũ����ʻ�ɫ | |
| D�� | CaCO3��ˮ�е��ܽ�Ⱥ�С����CaCO3Ϊ������� |
| A�� | NaHCO3�TNa++H++CO32- | B�� | NaHSO4�TNa++H++SO42- | ||
| C�� | Al2��SO4��3�TAl3++SO42- | D�� | NH3•H2O�TNH4++OH- |
| A�� | ������ǰ�ڣ���������ˮʱ����ClO2��Ϊ������ | |
| B�� | �������ڼ䣬���ÿɽ���ġ��������ϡ����һ���Է��У��ɷ�ֹ������ɫ��Ⱦ | |
| C�� | ����ͣ������װ�������ʩ���ɽ�����β���е�CO��NO��Ӧ���������� | |
| D�� | �������й���һ������֮�ڡ�ʹ�õĸֽ���������ڸ߷��ӻ����� |
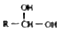 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$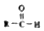 �������ͼ�ش�
�������ͼ�ش�
 ��
�� ����CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
����CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��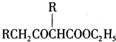 +C2H3OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����
+C2H3OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����