��Ŀ����
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2��������ͼ��ʾװ�ý���ʵ�飨����̨�����С��ƾ��Ƶ�δ����������ش��й����⣺
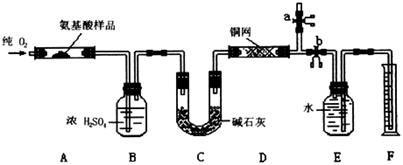
��1��ʵ�鿪ʼʱ�����ȴ�ֹˮ��a���ر�ֹˮ��b��ͨһ��ʱ��Ĵ�������������Ŀ���� ��֮������ر�ֹˮ�� ����ֹˮ�� ��
��2������װ������Ҫ���ȵ��У���װ�ô��ţ� ������ʱӦ�ȵ�ȼ ���ľƾ��ơ�
��3��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4��װ��D�������� ��
��5��Ϊ��N2����ˮ�����������ӦǰӦ������E��Fװ�õIJ��������г��� ��ˮ������������������������ ��
��6����ȡN2����ˮ�����ʱ��Ҫע�⣺�� ��
�� ��
��7��ʵ���в��N2�����ΪV mL���ѻ���ɱ�״������Ϊȷ���˰�����ķ���ʽ������õ��������У�����ĸ�� ��
A.���ɶ�����̼��������� B.����ˮ������
C.ͨ����������� D.�ð������Ħ������
��8�������װ����B��C������˳���ΪC��B����ʵ���Ŀ���ܷ�ﵽ���������� ��
��1����װ���е�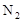 �ž���a��b����2��A��D��D
�ž���a��b����2��A��D��D
��3��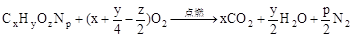
��4������δ��Ӧ��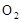 ����֤�����ռ����������Ƿ�Ӧ���ɵ�
����֤�����ռ����������Ƿ�Ӧ���ɵ�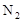 ��
��
��5��ˮ����β���������������壬�����ܱ��ռ��Ͳ��������½ϴ��ʵ����������ˮʱ����֤����װ��E������������ų�ˮ�����������ȡ�
��6������Ͳ�е�Һ��Ӧ����ƿ�е�Һ�����ƽ��������Ӧ��̶��ߺͰ�Һ����͵����С�
��7��A��B��D
��8�����ܴﵽ��ʵ���Ŀ�ġ���Ϊ��ʯ���Ǽ��Ը��������ͬʱ����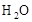 ��
��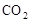 �������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɡ�
�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɡ�
��������
�����������1������װ���к��п������������к��е����������ʵ�顣����Ŀ�ľ��ǽ�װ���е�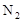 �ž�����ֹ���ţ����ʵ����Ȼ��Ϳ��Թر�ֹˮ��a����ֹˮ��b��
�ž�����ֹ���ţ����ʵ����Ȼ��Ϳ��Թر�ֹˮ��a����ֹˮ��b��
��2��������������ķ�Ӧ���Լ�ͭ���������ķ�Ӧ����Ҫ���ȣ���ѡA��D��Ϊ�˷�ֹ���������ս���E�У���������Ҫ��ȼD���ƾ��ơ�
��3���������������Ӧ�Ļ�ѧ����ʽ��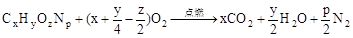 ��
��
��4���������Ϸ�����֪��ͭ��������������δ��Ӧ�� ����֤�����ռ����������Ƿ�Ӧ���ɵ�
����֤�����ռ����������Ƿ�Ӧ���ɵ� ��
��
��5����β���������������壬�����ܱ��ռ��Ͳ��������½ϴ��ʵ����������ˮʱ����֤����װ��E������������ų�ˮ�����������ȣ�����Ϊ��N2����ˮ�����������ӦǰӦ������E��Fװ�õIJ��������г���ˮ��
��6������ʱ���뱣֤ѹǿ��ͬ������ע�������Ǣ���Ͳ�е�Һ��Ӧ����ƿ�е�Һ�����ƽ���������Ӧ��̶��ߺͰ�Һ����͵����С�
��7������ͨ����������ֱ�ͭ��Ӧ���������������������Ҫ�����ݷ�Ӧ�Ļ�ѧ����ʽ��֪��Ҫ�ⶨ������Ļ�ѧʽ������������غ㶨�ɿ�֪������Ҫ�����������ɶ�����̼���������������ˮ�������Լ��ð������Ħ����������ѡA��B��D��
��8����Ϊ��ʯ���Ǽ��Ը��������ͬʱ���� ��
��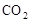 �������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɣ����Բ��ܶԵ���
�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɣ����Բ��ܶԵ���
���㣺���鰱���ữѧʽ�ⶨ���й�ʵ���жϡ�ʵ�����ۡ�����Լ�ʵ�����ݴ�����
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���ѵ�ϴ�ѧ�����ۺ���������˸��ߵ�Ҫ��������Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ���������������ѧ������˼ά�����ͷ�ɢ˼ά�������ر���ѧ����ʵ����ơ����������۵��ۺ�������Ҳ����������ѧ����ѧϰ��Ȥ�����ѧ����ѧϰЧ�ʡ�





