��Ŀ����
��1��0.1molH2O������Ϊ______g�����к�����______ ��ˮ���ӣ���ԭ�ӵ����ʵ���Ϊ______����2��������ͬ��H2��NH3��SO2��O3���������У����з�����Ŀ���ٵ���______������ͬ�¶Ⱥ���ͬѹǿ�����£����������______��
��3����������Ϊ49%��������Һ�����ܶ�Ϊ1.8g/cm3���������ʵ���Ũ��Ϊ______mol?L-1��
��4����12gij���۽������������У�������0.4mol��ԭ�ӣ��������ε�Ħ������Ϊ______g?mol-1
��5���������ӵķλ���ԼΪ3500��4000mL������Ů�ӵķλ���ԼΪ2500��3500mL���λ����ϴ��������λ�����С��Ů����������������ʵ���֮��ԼΪ����ͬ��ͬѹ�£�______��
��2������N=nNA=
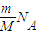 ֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�����V=
֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�����V=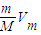 ֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�
֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ���3��C=
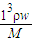 ��
����4�����������ε����ʵ�������ԭ�ӵ����ʵ���֮��Ĺ�ϵʽ���������ε�Ħ��������
��5����ͬ�����£���������ʵ���֮�ȵ��������֮�ȣ�
����⣺��1��m=nM=0.1mol×18g/mol=1.8g��N=nNA=0.1mol×NA/mol=0.1NA��һ��ˮ�����к���2����ԭ�ӣ�������ԭ�ӵ����ʵ�����ˮ��2��������ԭ�ӵ����ʵ�����0.2mol���ʴ�Ϊ��1.8��0.1NA��0.2mol��
��2������N=nNA=
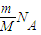 ֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�������ͬ��H2��NH3��SO2��O3���������У�Ħ�������������庬�еķ��������٣�Ħ���������������Ƕ����������Է��������ٵ��Ƕ�������
֪����ͬ���������壬������Ŀ��Ħ�������ɷ��ȣ�������ͬ��H2��NH3��SO2��O3���������У�Ħ�������������庬�еķ��������٣�Ħ���������������Ƕ����������Է��������ٵ��Ƕ�����������V=
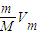 ֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�������������Ħ��������С��Ħ��������С����������
֪����ͬ��������ͬ���������壬���������Ħ�������ɷ��ȣ�������������Ħ��������С��Ħ��������С�����������ʴ�Ϊ��SO2��H2��
��3��C=
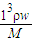 =
=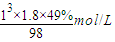 =9mol/L��
=9mol/L���ʴ�Ϊ��9��
��4��1molij���۽������������к���4mol��ԭ�ӣ�����Ϊ0.4mol��ԭ�ӵ������ε����ʵ�����0.1mol��M=
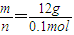 =120g/mol���ʴ�Ϊ��120��
=120g/mol���ʴ�Ϊ��120�� ��5����ͬ�����µ�����Ħ�������ͬ������V=nVmZ֪����������֮�ȵ������ʵ���֮�ȣ������ϴ��������λ�����С��Ů����������������ʵ���֮��ԼΪ����ͬ��ͬѹ�£�=4000��2500=8��5���ʴ�Ϊ��8��5��
���������⿼�������ʵ����ļ��㣬Ũ�Ȳ������ջ�����ʽ�ǽⱾ��Ĺؼ����й���������ļ�����Ҫע���¶Ⱥ�ѹǿ��Ϊ�״��㣮

�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�
��֪��
�� R1CH=CHR2  R1COOH + R2COOH ��R1��R2����������
R1COOH + R2COOH ��R1��R2����������
 |
��
��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ���������������������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ��ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH����������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�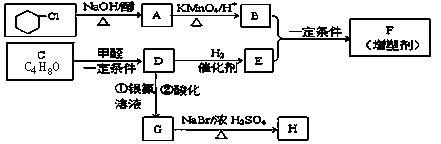
��֪���� ��R1��R2����������
��R1��R2����������
|
 ��C�ܷ���������Ӧ���ҷ�������֧����
��C�ܷ���������Ӧ���ҷ�������֧���� ��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ�������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ�
�ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH
���� ������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�

��֪�� �� ��R1��R2����������
��R1��R2����������
|

��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ�������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ�
�ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH
���� ������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�л��ϳ����ִ�����ũҵ������ռ���൱��Ҫ�ĵ�λ���л���F��һ�ָ߷��ӻ�����������ܼ���H�dz��ݼ����м������ǵĺϳ�·�����£�

��֪��
�� R1CH=CHR2  R1COOH + R2COOH
��R1��R2����������
R1COOH + R2COOH
��R1��R2����������
 |
��
 |
��C�ܷ���������Ӧ���ҷ�������֧����
��ش�
��1��E�������������ֻ�ѧ������ͬ��Hԭ�ӣ�ԭ�Ӹ�������������������
��2��D���������������ŵ������ǣ���������������������������������������
��3��д����һ�Ȼ����鵽A�Ļ�ѧ����ʽ��������������������������������������������
��4��д����������������D��һ��ͬ���칹��Ľṹ��ʽ ��
����D������ͬ�Ĺ����ţ��ڷ����о�����������̼ԭ��(�����ĸ���ͬԭ�ӻ���ŵ�̼ԭ�ӣ���Ϊ����̼ԭ��)��
��5��G��H����Է����������63��H����NaHCO3��Һ��Ӧ����0.1moLH������NaOH��Һ��Ӧ������NaOH����������������moL��
��6��B��E��һ������������F�ķ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��



