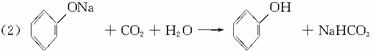��Ŀ����
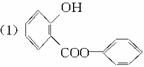
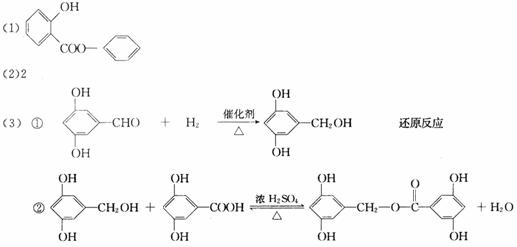
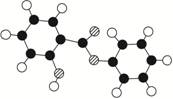
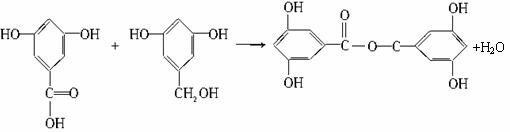
����(Salol)��һ�������������ķ���ʽΪC13H10O3,�����ģ������ͼ��ʾ(ͼ��������֮������ߴ�����ѧ�����絥����˫����)��
(1)������ͼģ��д�����Ľṹ��ʽ��___________��
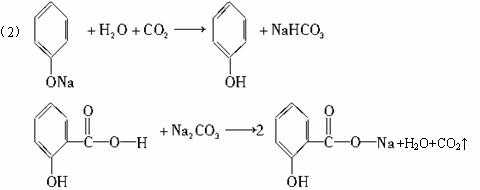
(2)����ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ����(���ǻ�������)�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ(�û�ѧ����ʽ��ʾ)
_____________________________________________________________
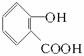
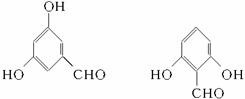
(3)ͬʱ��������Ҫ���ˮ�����ͬ���칹�干���֡�
�ٺ��б��������ܷ���������Ӧ������ϡNaOH��Һ�У�1mol��ͬ���칹������2molNaOH��Ӧ;��ֻ����������һ�ȴ��
(4)��(3)ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊ���п�ͼ�е�A��

д������������Ӧ�Ļ�ѧ����ʽ(ˮ�����ýṹ��ʽ��ʾ)����ָ����Ӧ�ķ�Ӧ���͡�
��A��B___________________________________________________
��Ӧ����_________��
��B+D��E_________________________________________________
��Ӧ����_________��
(5)����ˮ�����뱽�ӵĻ������ǵ����ʵ���֮��Ϊnmol���û������ȫȼ������O2aL��������bgH2O,cLCO2(���������Ϊ��״���µ����)
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ(�л�����÷���ʽ��ʾ)
___________________________________________________________
___________________________________________________________
����������ˮ��������ʵ���Ϊxmol���г�x�ļ���ʽ��
������(1)���ݽṹģ��ͼ����֪������ɫ����̼����ɫ�����⣬��ɫ������������ṹ��ʽΪ��
C6H5��OOC��C6H4��OH��
(2)CO2+C6H5ONa+H2O��C6H5OH+NaHCO3
2HO��C6H4��COOH+Na2CO3��2HO��C6H4��COONa+H2O+CO2��
(3)2�֣��ֱ��Ǽ�λ���λ��

(4)A��B��

�ӳɷ�Ӧ��
B+D��E��
������Ӧ(ȡ����Ӧ)��
(5)C7H6O3+7O2��7CO2+3H2O��C6H6O+7O2��6CO2+3H2O
x=c/22.4-6n(n=b/54,n=a/156.8)
�𰸣�(1)C6H5��OOC��C6H4��OH
(3)2
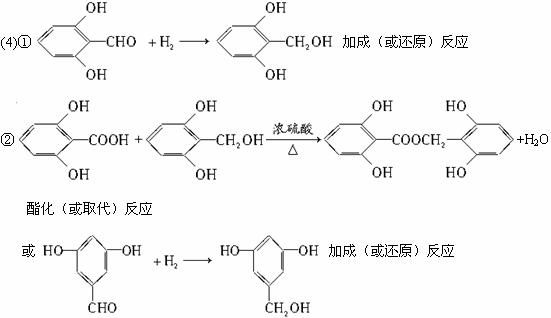

������(��ȡ��)��Ӧ
(5)��![]()
![]()
��x=c/22.4-6n����x=c/22.4-b/9

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�