��Ŀ����
������һ��ǿ������������������������ȡ�
(1)O3��KI��Һ��Ӧ���ɵ����ֵ�����________��________(�����ʽ)��
(2)O3��ˮ���ֽ⣬һ�������£�O3��Ũ�ȼ���һ�������ʱ��(t)���±���ʾ����֪��O3����ʼŨ��Ϊ0.0216 mol/L��

��pH�����ܼ���O3�ֽ⣬������O3�ֽ�������õ���________��
����30�桢pH��4.0�����£�O3�ķֽ�����Ϊ________mol/(L��min)��
�۾ݱ��еĵݱ���ɣ��Ʋ�O3�����������·ֽ��������������˳��Ϊ________(����ĸ����)��
a��40�桢pH��3.0������ b��10�桢pH��4.0
c��30�桢pH��7.0
(3)O3���ɳ���������(ԭ����ͼ)���ϡ�����Ƶá�
��ͼ������Ϊ________(�A����B��)����缫��ӦʽΪ_______________________��
����C��ͨ��O2����A���ĵ缫��ӦʽΪ________��
����C����ͨ��O2��D��E���ֱ��ռ���x L��y L����(��״��)����E���ռ���������O3��ռ���������Ϊ________(����O3�ķֽ�)��

(1)O3��KI��Һ��Ӧ���ɵ����ֵ�����________��________(�����ʽ)��
(2)O3��ˮ���ֽ⣬һ�������£�O3��Ũ�ȼ���һ�������ʱ��(t)���±���ʾ����֪��O3����ʼŨ��Ϊ0.0216 mol/L��

��pH�����ܼ���O3�ֽ⣬������O3�ֽ�������õ���________��
����30�桢pH��4.0�����£�O3�ķֽ�����Ϊ________mol/(L��min)��
�۾ݱ��еĵݱ���ɣ��Ʋ�O3�����������·ֽ��������������˳��Ϊ________(����ĸ����)��
a��40�桢pH��3.0������ b��10�桢pH��4.0
c��30�桢pH��7.0
(3)O3���ɳ���������(ԭ����ͼ)���ϡ�����Ƶá�
��ͼ������Ϊ________(�A����B��)����缫��ӦʽΪ_______________________��
����C��ͨ��O2����A���ĵ缫��ӦʽΪ________��
����C����ͨ��O2��D��E���ֱ��ռ���x L��y L����(��״��)����E���ռ���������O3��ռ���������Ϊ________(����O3�ķֽ�)��

(1)I2��O2
(2)��OH������1.00��10��4����b��a��c
(3)��A��2H����2e��=H2��
��O2��4H����4e��=2H2O����(x��2y)/y
(2)��OH������1.00��10��4����b��a��c
(3)��A��2H����2e��=H2��
��O2��4H����4e��=2H2O����(x��2y)/y
�����Գ���Ϊ���忼���˻�ѧ��Ӧ���ʡ��绯ѧ�Ȼ�ѧ��Ӧԭ����ͬʱ�����˿�����ͼ���Ĺ۲����������
(1)�������������ԣ�����⻯�ط���������ԭ��Ӧ�����������ⵥ�ʺ��������ء�
(2)30�桢pH��4.0ʱ�������ֽ�һ������ʱ��Ϊ108 min����Ӧ����v��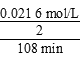 ��1.00��10��4mol/(L��min)����ϱ�������֪��Ӧ������b��������С��c���������
��1.00��10��4mol/(L��min)����ϱ�������֪��Ӧ������b��������С��c���������
(3)�۲�绯ѧװ��ͼ֪��������Ե缫B�ϲ����������ͳ������ü�ʧȥ���ӷ���������Ӧ����������������Ե缫AΪ�������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H����2e��=H2��������C��ͨ�����������������뷴Ӧ���������ɵĽ���ˮ���缫��ӦΪO2��4H����4e��=2H2O����y L��������г������������Ϊa���ɵ����غ���2x��y a��6��y(1��a)��4�����a��(x��2y)/y��
(1)�������������ԣ�����⻯�ط���������ԭ��Ӧ�����������ⵥ�ʺ��������ء�
(2)30�桢pH��4.0ʱ�������ֽ�һ������ʱ��Ϊ108 min����Ӧ����v��
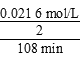 ��1.00��10��4mol/(L��min)����ϱ�������֪��Ӧ������b��������С��c���������
��1.00��10��4mol/(L��min)����ϱ�������֪��Ӧ������b��������С��c���������(3)�۲�绯ѧװ��ͼ֪��������Ե缫B�ϲ����������ͳ������ü�ʧȥ���ӷ���������Ӧ����������������Ե缫AΪ�������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H����2e��=H2��������C��ͨ�����������������뷴Ӧ���������ɵĽ���ˮ���缫��ӦΪO2��4H����4e��=2H2O����y L��������г������������Ϊa���ɵ����غ���2x��y a��6��y(1��a)��4�����a��(x��2y)/y��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 2ClO2����K2SO4��2CO2����2H2O������˵������ȷ����
2ClO2����K2SO4��2CO2����2H2O������˵������ȷ����

 H2O2+BaCl2
H2O2+BaCl2 2H2O+O2��
2H2O+O2��



 Al2O3��2Fe��1��2
Al2O3��2Fe��1��2