��Ŀ����
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
CH3CH=CH2+CO+H2![]() CH3CH2CH2CHO
CH3CH2CH2CHO![]() CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��
CO���Ʊ�ԭ����HCOOH![]() CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã���ͼ����
CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã���ͼ����
����д���пհף�
��ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2-����������ѡ����ʵ��Լ��Ʊ���������ϩ��д����ѧ��Ӧ����ʽ�� �� ��
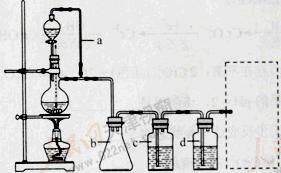
����������װ���Ʊ����﴿����CO��װ����a��b�����÷ֱ��� ��
��c��d��ʢװ���Լ��ֱ��� �� ����������װ���Ʊ�H2�����巢��װ���б���IJ������������� �������߿��ڻ����ռ�����H2��װ��ͼ��
���Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳���� ������ţ���
�ٱ���Na2SO3��Һ ������KMnO4��Һ ��ʯ��ˮ ����ˮCuSO4 ��Ʒ����Һ
�Ⱥϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�����ΪӦ�ò��õ����˷�Ӧ������ ��
a.���¡���ѹ������ b.�ʵ����¶ȡ���ѹ������
c.���¡���ѹ������ d.�ʵ����¶ȡ���ѹ������
������ȩ��������õ�����������ȩ��1-������Ʒ��Ϊ����1-��������С���������֪����R-CHO+NaHSO3�����ͣ�![]() RCH(OH)SO3Na�����ڷе㣺����34�棬1-���� 118�棬����Ƴ������ᴿ·�ߣ�
RCH(OH)SO3Na�����ڷе㣺����34�棬1-���� 118�棬����Ƴ������ᴿ·�ߣ�
��Ʒ![]() ��Һ
��Һ ![]() �л���
��� ![]() 1-����������
1-���������� ![]() ��Ʒ
��Ʒ
�Լ�1Ϊ ������1Ϊ ������2Ϊ ������3Ϊ ��
��Zn+2HCl![]() ZnCl2+H2��
ZnCl2+H2�� ![]()
![]() CH3CH=CH2��+H2O
CH3CH=CH2��+H2O
�ƺ�ѹ ������ NaOH��Һ ŨH2SO4 ��Һ©����������ƿ 
�Ǣܢݢ٢ڢۣ���ܢݢ٢ۢڣ� ��b �ɱ���NaHSO3��Һ ���� ��ȡ ����
���������Ʊ�����ѡ��п����ϡ���ᣬ��Ӧ����ʽ���𰸣��Ʊ���ϩѡ��2-������Ũ���ᣬ��Ӧ����ʽ���𰸡��������װ���У�a�����ñ��ַ�Һ©������ƿ�ڵ���ѹ��ȣ��Ա�֤��Һ©���ڵ�Һ����˳��������ƿ�У�b��Ҫ����ȫƿ�����ã��Է�ֹ������cΪ��ȥCO�е��������壬ѡ��NaOH��Һ��dΪ��ȥCO�е�H2O���Լ�ѡ��Ũ����������װ���Ʊ�H2������Ҫ�ƾ��ơ��Ǽ����ϩ������SO2��CO2��ˮ������ɵĻ��������ɷ�ʱ��Ӧ����ѡ����ˮCuSO4����ˮ������Ȼ���â�Ʒ����Һ����SO2�����âٱ���Na2SO3��Һ��ȥSO2��Ȼ���â�ʯ��ˮ����CO2���â�����KMnO4��Һ�����ϩ��������ϳ�����ȩ�ķ�ӦΪ���������С�ķ��ȷ�Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�ѡb���ɼ��𰸡�

 ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����B���Ӧ�ij��������ʵ���Ϊ
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
�������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��Ӧ�����ӷ���ʽΪ
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
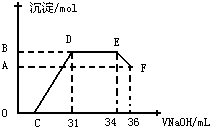
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ
��3��B���Ӧ�ij��������ʵ���Ϊ

 2Fe2+
+ I2Ϊ���淴Ӧ������Ӧ����һ�����ȣ���������¼��ַ�������֪FeF63-��һ����ɫ���ȶ��������ӡ�
2Fe2+
+ I2Ϊ���淴Ӧ������Ӧ����һ�����ȣ���������¼��ַ�������֪FeF63-��һ����ɫ���ȶ��������ӡ�
 2Fe2+ + I2ƽ�⳯�淴Ӧ�����ƶ���������Һ��ɫ���dz��
2Fe2+ + I2ƽ�⳯�淴Ӧ�����ƶ���������Һ��ɫ���dz��