��Ŀ����
��1�����ʵ���Ũ����ͬ��������Һ����Na2CO3����NaHCO3����H2CO3���ܣ�NH4��2CO3����NH4HCO3��[CO32-]��С�������е�˳����
��A���ݣ��ܣ��ۣ��ڣ��٣�B���ۣ��ݣ��ڣ��ܣ���
��C���ۣ��ڣ��ݣ��ܣ��٣�D���ۣ��ݣ��ܣ��ڣ���
��2��д0.2mol/L Na2CO3��Һ�еĵ���غ�ʽ �������غ�ʽ �������غ�ʽ ��
��3��д�����ӷ���˫ˮ������ӷ���ʽ��ͬʱ�������Ӳ��ܹ����ԭ��
Al3+��CO32- Fe3+��HCO3-
��4����6��0.01mol/L��ˮ�зֱ�������и����ʣ�A��Ũ��ˮ B����ˮ C������K2CO3 D������H2SO4 E������NaOH���� F������Al2��SO4��3����
?��ʹc��OH-����С��c��NH4+���������
?��ʹc��OH-������c��NH4+����С����
?��ʹc��OH-����c��NH4+����������� ��
��A���ݣ��ܣ��ۣ��ڣ��٣�B���ۣ��ݣ��ڣ��ܣ���
��C���ۣ��ڣ��ݣ��ܣ��٣�D���ۣ��ݣ��ܣ��ڣ���
��2��д0.2mol/L Na2CO3��Һ�еĵ���غ�ʽ
��3��д�����ӷ���˫ˮ������ӷ���ʽ��ͬʱ�������Ӳ��ܹ����ԭ��
Al3+��CO32-
��4����6��0.01mol/L��ˮ�зֱ�������и����ʣ�A��Ũ��ˮ B����ˮ C������K2CO3 D������H2SO4 E������NaOH���� F������Al2��SO4��3����
?��ʹc��OH-����С��c��NH4+���������
?��ʹc��OH-������c��NH4+����С����
?��ʹc��OH-����c��NH4+�����������
���㣺����Ũ�ȴ�С�ıȽ�,����ˮ���Ӧ��
ר�⣺�����ˮ��ר��
��������1��H2CO3Ϊ��Ԫ���ᣬ���ȶ��������ֽ⣻
Na2CO3��ǿ�������Σ�ˮ��ʼ��ԣ�
NaHCO3��ǿ�������ε���ʽ�Σ�ˮ��ʼ��ԣ���ˮ��̶ȱ�̼����С�����в��ȶ��ԣ�
��NH4��2CO3�����������Σ�������ٽ���ˮ�⣬���в��ȶ��ԣ�
NH4HCO3��Һ���������������ʽ�Σ�������ٽ���ˮ�⣬���в��ȶ��ԣ�
��2���κε������Һ�ж����ڵ���غ㡢�����غ�������غ㣻
��3��Al3+��CO32-��ٽ�ˮ���������������Ͷ�����̼��Fe3+��HCO3-����˫ˮ�����������Ͷ�����̼��
��4����ˮ�д���NH3��H2O?NH4++OH-��
�������ǿ����������ʹc��OH-����С��c��NH4+������
������ǿ����������ʹc��OH-������c��NH4+����С��
?����Ũ��ˮ�������¶���ʹc��OH-����c��NH4+��������
Na2CO3��ǿ�������Σ�ˮ��ʼ��ԣ�
NaHCO3��ǿ�������ε���ʽ�Σ�ˮ��ʼ��ԣ���ˮ��̶ȱ�̼����С�����в��ȶ��ԣ�
��NH4��2CO3�����������Σ�������ٽ���ˮ�⣬���в��ȶ��ԣ�
NH4HCO3��Һ���������������ʽ�Σ�������ٽ���ˮ�⣬���в��ȶ��ԣ�
��2���κε������Һ�ж����ڵ���غ㡢�����غ�������غ㣻
��3��Al3+��CO32-��ٽ�ˮ���������������Ͷ�����̼��Fe3+��HCO3-����˫ˮ�����������Ͷ�����̼��
��4����ˮ�д���NH3��H2O?NH4++OH-��
�������ǿ����������ʹc��OH-����С��c��NH4+������
������ǿ����������ʹc��OH-������c��NH4+����С��
?����Ũ��ˮ�������¶���ʹc��OH-����c��NH4+��������
���
�⣺��1���٢���Ƚϣ����ڢܷ�������ˮ�⣬ˮ��̶Ƚϴ���٣��ܣ��ڢ���Ƚϣ��ݷ�������ˮ�⣬c��CO32-���Ĵ�СΪ�ڣ��ݣ���Ϊ��Ԫ���ᣬc��CO32-����С����c��CO32-���Ĵ�С��ϵΪNa2CO3����NH4��2CO3��NaHCO3��NH4HCO3��H2CO3����ѡB��
��2����Һ�д��ڵ���غ�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-����
�����غ��ϵ��Ϊc��Na+��=2[c��HCO3-��+c��CO32-��+c��H2CO3��]��
�����غ�ʽΪc��OH-��=c��HCO3-��+2c��H2CO3��+c��H+����
�ʴ�Ϊ��c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-����c��Na+��=2[c��HCO3-��+c��CO32-��+c��H2CO3��]��c��OH-��=c��HCO3-��+2c��H2CO3��+c��H+����
��3��Al3+��CO32-��ٽ�ˮ���������������Ͷ�����̼�����ӷ���ʽΪ2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����Fe3+��HCO3-����˫ˮ�����������Ͷ�����̼�����ӷ���ʽΪ Fe3++3HCO3-=Fe��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����Fe3++3HCO3-=Fe��OH��3��+3CO2����
��4����ˮ�д���NH3��H2O?NH4++OH-��
���������������������������ˮ����������������ʹƽ���������ƶ�����c��OH-�����١�c��NH4+������ѡDF��
��ʹc��OH-������c��NH4+�����٣�Ӧ������ˮ��ʼ��Ե����ʣ���NaOH��Һ�Լ�����K2CO3���ϣ���ѡ��CE��
����Ũ��ˮ����ʹc��OH-����c��NH4+��������ѡ��A��
��2����Һ�д��ڵ���غ�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-����
�����غ��ϵ��Ϊc��Na+��=2[c��HCO3-��+c��CO32-��+c��H2CO3��]��
�����غ�ʽΪc��OH-��=c��HCO3-��+2c��H2CO3��+c��H+����
�ʴ�Ϊ��c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-����c��Na+��=2[c��HCO3-��+c��CO32-��+c��H2CO3��]��c��OH-��=c��HCO3-��+2c��H2CO3��+c��H+����
��3��Al3+��CO32-��ٽ�ˮ���������������Ͷ�����̼�����ӷ���ʽΪ2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����Fe3+��HCO3-����˫ˮ�����������Ͷ�����̼�����ӷ���ʽΪ Fe3++3HCO3-=Fe��OH��3��+3CO2����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����Fe3++3HCO3-=Fe��OH��3��+3CO2����
��4����ˮ�д���NH3��H2O?NH4++OH-��
���������������������������ˮ����������������ʹƽ���������ƶ�����c��OH-�����١�c��NH4+������ѡDF��
��ʹc��OH-������c��NH4+�����٣�Ӧ������ˮ��ʼ��Ե����ʣ���NaOH��Һ�Լ�����K2CO3���ϣ���ѡ��CE��
����Ũ��ˮ����ʹc��OH-����c��NH4+��������ѡ��A��
���������⿼��������Ũ�ȴ�С�Ƚϡ�����ƽ���ƶ���֪ʶ�㣬������Һ�е����ʼ�������ȷ����Һ������Ũ�ȴ�С���ٽ����������Ի�ѧƽ���Ӱ����ȷ����Һ������Ũ�ȱ仯����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
���nΪ�ڢ�A����XԪ�ص�ԭ����������ԭ������Ϊ��n+2����YԪ�ؿ���λ�ڣ�������
�٢�A �ڢ�A�� ���ۢ�A�� ���ܢ�B �ݢ�B�������ޢ�B��
�٢�A �ڢ�A�� ���ۢ�A�� ���ܢ�B �ݢ�B�������ޢ�B��
| A���٢� | B���ڢ� | C���ۢ� | D���ڢ� |
����˵��������ǣ�������
| A��Ũ��������ڸ���O2��CO2 |
| B��ϡ���������Ӧ�ɲ������� |
| C�����ɽ�ˮֱ�ӵ���Ũ���������ϡ�� |
| D���������Ż�ʱ������ˮ��Ϩ�� |

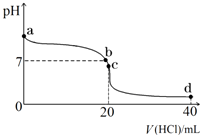 �����£�����0.1mol?L-1�������20mL 0.1mol?L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ��
�����£�����0.1mol?L-1�������20mL 0.1mol?L-1��ˮ�У���ҺpH�������������ı仯������ͼ��ʾ�� ��1�����ӱ��еĵ�Դ��������пԭ��أ���缫�ֱ�ΪAg2O��Zn���������ҺΪKOH��Һ���ܷ�ӦʽΪ��Ag2O+H2O+Zn�TZn��OH��2+2Ag����ش𣺷ŵ�ʱ�������缫�ϵķ�ӦʽΪ��
��1�����ӱ��еĵ�Դ��������пԭ��أ���缫�ֱ�ΪAg2O��Zn���������ҺΪKOH��Һ���ܷ�ӦʽΪ��Ag2O+H2O+Zn�TZn��OH��2+2Ag����ش𣺷ŵ�ʱ�������缫�ϵķ�ӦʽΪ��