��Ŀ����
|
����ȫ�������Ա��ͨ��������������ʽȷ�ϡ�SARS����һ�ֹ�״�������죬�ò������Ŵ�������RNA�������йء�SARS�������������д������ | |
| [����] | |
A�� |
��SARS������Ҫ����;���Ǽ�ӽӴ����� |
B�� |
��SARS��������һ�ֶ��ﲡ�� |
C�� |
��SARS��������������������Сʱ���������ڻ�ϸ�������� |
D�� |
��SARS������ֻ�е�����û�к��� |
�𰸣�D
������
������
|
�����ϵ�����һ�㶼�����������ʵ����ʺͺ��ᣬ��������ֻ���к��ᣬ���Dz�����ֻ���е����ʲ����������� |

��ϰ��ϵ�д�
�����Ŀ

 ����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ
����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ
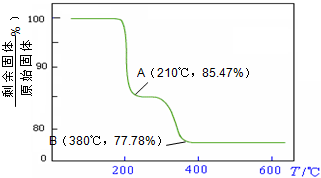 ��NH4VO3�ڷֽ������
��NH4VO3�ڷֽ������
