��Ŀ����
��10�֣������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����

�ش��������⣺
��1��������Ӧ���������������� (����)��
��2����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,��̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ:
�������������������������������������� ��������������������������������������
��Һ��������������������������������������
��3����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ ��
(1) 4 8
(2 )2H++2e-=H2�� Fe-2e-=Fe2+ Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
(3)2CrO42-+2H+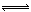 Cr2O72-+H2O
Cr2O72-+H2O
�����������⿼����������ԭ��Ӧ�����ӷ�Ӧ���绯ѧ�����֪ʶ��
��1�����ݸ��Ļ��ϼۿ�֪�����ϼ����ߵķ�Ӧ��Ȼ��������ṩ������
��2��������Ҫʧ���� Fe-2e-=Fe2+���ٸ������ӵķŵ�˳��֪��H+�������ŵ�2H++2e-=H2�� ��
Fe2+�IJ����ṩ�˻�ԭ������Cr2O72-������Cr2O72- + 6Fe2+ + 14H+ = 2Cr3+ +6Fe3++7H2O
��3����Ҫ�������ӷ���ʽ����д����ƽ


 Cr2O72-+H2O
Cr2O72-+H2O ��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����
��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ���� Cr2O72-+H2O
Cr2O72-+H2O
 Cr2O72-+H2O
Cr2O72-+H2O