��Ŀ����
����Ŀ��H2C2O4Ϊ��Ԫ���ᡣ20������һ��c(H2C2O4)+c(HC2O4�C)+c(C2O42�C)=0.100 mol��L�C1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ��һ����ȷ����
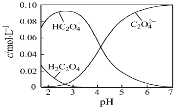
A��pH=2.5����Һ�У�c(H2C2O4)+c(C2O42�C)��c(HC2O4�C)
B��c(Na+)=0.100 mol��L�C1����Һ�У�c(H+)+c(H2C2O4)=c(OH�C)+c(C2O42�C)
C��c(HC2O4�C)=c(C2O42�C)����Һ�У�c(Na+)��0.100 mol��L�C1+c(HC2O4�C)
D��pH=7����Һ�У�c(Na+)<2c(C2O42�C)
���𰸡�B
��������
���������A������ͼ���֪��PH=2.5����Һ��c(H2C2O4)��c(C2O42-)Ũ��֮��С��c(HC2O4-)����c(H2C2O4)+c(C2O42-)��c(HC2O4-)����A����B��������Һ�е���غ�������غ������c(Na+)=0.100mol/L����Һ��ΪNaHC2O4��Һ����Һ�д��ڵ���غ�(H+)+c(Na+)=2c(C2O42-)+c(HC2O4-)+c(OH-)�������غ�c(Na+)=c(C2O42-)+c(HC2O4-)+c(H2C2O4)���������õ�c(H+)+c(H2C2O4)=c(OH-)+c(C2O42-)����B��ȷ��C��c(H2C2O4)+c(HC2O4-)+c(C2O42-)=0.100molL-1��c(HC2O4-)=c(C2O42-)������غ�(H+)+c(Na+)=2c(C2O42-)+c(HC2O4-)+c(OH-)��pHԼ4����ʱ������Ũ�ȴ������������õ���Һ��c(Na+)��0.100 molL-1+c(HC2O4-)����C����D��pH=7�����ݵ���غ�(H+)+c(Na+)=2c(C2O42-)+c(OH-)����������ˮ�⣬����c(Na+)��2c(C2O42-)����D��ȷ����ѡB��



