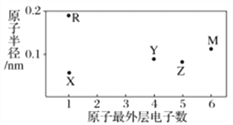
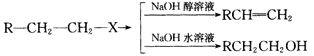
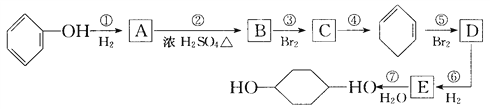
��Ŀ����
����Ŀ�����������������ʣ���KNO3����Һ̬HCl����ͭ˿����NaHCO3����Fe(OH)3��������CO2
��1���ټ��Ǽ������������Σ��Ը����ʵķ������___________________________��
��2���Ȳ��ǵ����Ҳ���Ƿǵ���ʵ��ǣ�����ţ�___________��
��3��ʵ�����Ʊ�Fe(OH)3����Ļ�ѧ����ʽΪ________________________�����ٺ�ɫ������(Sb2S3)����װ��U�ܣ�����缫��ֱͨ���磬�������������ٺ�ɫ���֤��Sb2S3������_____��ɡ�Fe(OH)3�����ེ���ϣ�������ϵ����ǣ����Ƿ�����___________��
��4��θ���к������ᣬNaHCO3������θ����࣬��д���䷴Ӧ�����ӷ���ʽ_____________��������θ����ʱ��÷��ú�Al(OH)3��θҩ������θ�ᷴӦ�����ӷ���ʽΪ______________������ʯ��ˮ��ͨ�������CO2��ʱ������Ӧ�����ӷ���ʽ��______________________________��
���𰸡�������෨ �ۢ� FeCl3+ 3H2O![]() Fe(OH)3(����)+3HCl �� ����۳� H+ +HCO3- = CO2 + H2O 3H+ +Al(OH)3 =Al3+ + 3H2O Ca(OH)2 + 2CO2 = Ca2++2HCO3-
Fe(OH)3(����)+3HCl �� ����۳� H+ +HCO3- = CO2 + H2O 3H+ +Al(OH)3 =Al3+ + 3H2O Ca(OH)2 + 2CO2 = Ca2++2HCO3-
��������
��1���ɽ�����෨��֪��KNO3���Ǽ������������Σ�
��2���������������ڵ���ʵ���KNO3��Һ̬HCl��NaHCO3�����ڷǵ���ʵ���CO2���Ȳ��ǵ����Ҳ���Ƿǵ���ʵ���ͭ˿��Fe(OH)3���壻
��3��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ����������ٺ�ɫ���˵��Sb2S3����������ɣ����෴���Fe(OH)3�����ེ���ϻᷢ���۳���
��4��θ���к������ᣬ��������NaHCO3��Al(OH)3��Ӧ������θ����ࣻ���������������̼��Ӧ���������Ե�̼��ƣ�ͨ������Ķ�����̼��������̼��̼��ƺ�ˮ��Ӧ����������ˮ��̼����ƣ����DZ���塣
��1���ɽ�����෨��֪��KNO3���Ǽ������������Σ��ʴ�Ϊ��������෨��
��2���������������ڵ���ʵ���KNO3��Һ̬HCl��NaHCO3�����ڷǵ���ʵ���CO2��ͭ˿�ǽ������ʡ�Fe(OH)3�����ǻ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ��ʴ�Ϊ���ۢݣ�
��3��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ���Ӧ�Ļ�ѧ����ʽΪFeCl3+ 3H2O![]() Fe(OH)3(����)+3HCl������缫��ֱͨ���磬�������������ٺ�ɫ���˵��Sb2S3����������ɣ�Fe(OH)3�����ེ���ϣ�������ɵ�Fe(OH)3������������Sb2S3���������۳�����ϵ����ǣ��ʴ�Ϊ��FeCl3+ 3H2O
Fe(OH)3(����)+3HCl������缫��ֱͨ���磬�������������ٺ�ɫ���˵��Sb2S3����������ɣ�Fe(OH)3�����ེ���ϣ�������ɵ�Fe(OH)3������������Sb2S3���������۳�����ϵ����ǣ��ʴ�Ϊ��FeCl3+ 3H2O![]() Fe(OH)3(����)+3HCl����������۳���
Fe(OH)3(����)+3HCl����������۳���
��4��θ���к������ᣬNaHCO3������θ����࣬����Ϊ������̼�����Ʒ�Ӧ�����Ȼ��ƺ�ˮ��������̼����Ӧ�����ӷ���ʽ��H++HCO3-=H2O+CO2�����������������ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽ��Al��OH��3+3H+=Al3++3H2O�������ʯ��ˮ��ͨ�������̼�����������������̼��Ӧ���������Ե�̼��ƣ�ͨ������Ķ�����̼��������̼��̼��ƺ�ˮ��Ӧ����������ˮ��̼����ƣ����DZ���壬��Ӧ�������ӷ���ʽΪ��Ca(OH)2 + 2CO2 = Ca2++2HCO3-���ʴ�Ϊ��H++HCO3-=H2O+CO2����Al��OH��3+3H+=Al3++3H2O��Ca(OH)2 + 2CO2 = Ca2++2HCO3-��