��Ŀ����
����1��50mol��L-1��NaOH��Һ100mL��
��1��ijѧ���������£�
����������ƽ�Ƴ�6��00g�������ƣ�����ƽ������㣬���������ϸ�ȡһ��ͬ��������ֽ�����������1��00g��λ���ϣ��������̷���״�������ƣ����̷�����������ƽƽ�⣬ȡ�³ƺõ��������ƣ������������ϵ�ֽ��
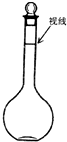
�ڰѳƺõ��������Ʒ���һֻ100mL���ձ��У�����Լ10mLˮ������ʹ֮�ܽ⣬�ܽ�������ò�������������Һ����һֻ100mL������ƿ�ڣ���ˮ����̶���Լ2cm�����õιܼ�ˮ���̶��ߡ�
��д��һ�������������ڵġ�0��25mol��L-1NaOH��Һ���ı�ǩ����������ƿ���ܱձ��档ָ�����������е�7������
��__________________________����_________________________
��__________________________����_________________________
��__________________________����_________________________
��__________________________
��2�����в������������ҺŨ��ƫ�ߵ���_______________
A������NaOH�Ѿ����⣻
B���ô������������ƽ��NaOHʱ�����ˡ������������
C��δ��ϴ�ܽ��õ��ձ��
D��δ��ȴ�����¾�ע������ƿ
E������ʱ�������ӿ̶��ߣ�
F��������ƽ������������
��1��ijѧ���������£�
����������ƽ�Ƴ�6��00g�������ƣ�����ƽ������㣬���������ϸ�ȡһ��ͬ��������ֽ�����������1��00g��λ���ϣ��������̷���״�������ƣ����̷�����������ƽƽ�⣬ȡ�³ƺõ��������ƣ������������ϵ�ֽ��
�ڰѳƺõ��������Ʒ���һֻ100mL���ձ��У�����Լ10mLˮ������ʹ֮�ܽ⣬�ܽ�������ò�������������Һ����һֻ100mL������ƿ�ڣ���ˮ����̶���Լ2cm�����õιܼ�ˮ���̶��ߡ�
��д��һ�������������ڵġ�0��25mol��L-1NaOH��Һ���ı�ǩ����������ƿ���ܱձ��档ָ�����������е�7������
��__________________________����_________________________
��__________________________����_________________________
��__________________________����_________________________
��__________________________
��2�����в������������ҺŨ��ƫ�ߵ���_______________
A������NaOH�Ѿ����⣻
B���ô������������ƽ��NaOHʱ�����ˡ������������
C��δ��ϴ�ܽ��õ��ձ��
D��δ��ȴ�����¾�ע������ƿ
E������ʱ�������ӿ̶��ߣ�
F��������ƽ������������
��1����NaOHӦ���ڸ�����ձ��г��������ù�������δ����ԭ������������ƽֻ�ܳƳ�0.1g���Ʋ���1.00g�����ձ��Ͳ�����δϴ�ӣ�ϴ��ҺҲӦת������ƿ�����ܽ�NaOHӦ��ȴ����ת��������ƿ�У�
������ƿ�е���Һδҡ�ȣ�����õ���ҺӦ��ʱת�Ƶ��н������Լ�ƿ��
��2��DEF
������ƿ�е���Һδҡ�ȣ�����õ���ҺӦ��ʱת�Ƶ��н������Լ�ƿ��
��2��DEF

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����100mL 0.50mol?L-1 NaOH��Һʱ�������õ��������ǣ�������
A��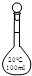 100mL����ƿ | B�� 100mL����ƿ | C�� 100mL��ƿ | D�� 100mL��ƿ |
 ʵ�������Ȼ��ƹ�������1.00mol/L��NaCl��Һ0.5L���ش���������
ʵ�������Ȼ��ƹ�������1.00mol/L��NaCl��Һ0.5L���ش���������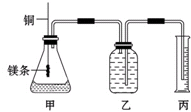 ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�