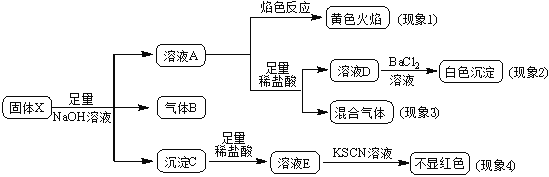
��Ŀ����
����Ŀ����ϩ����Ҫ���л�ԭ�ϣ��������ºϳ�·�ߣ��ش����⡣
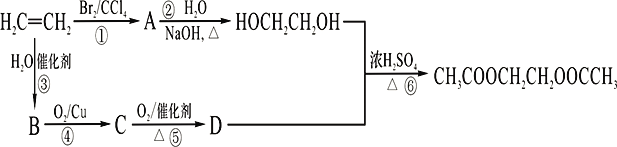
��1��д����ϩ�ĵ���ʽ_____________��A������Ϊ______________;
��2��B�����еĹ�����������_________________��
��3��д��ָ����Ӧ�ķ�Ӧ���ͣ���________________����______________��
��4��д��ָ����Ӧ�Ļ�ѧ����ʽ����________________����_____________��
��5����B��D��Ӧ�������ﻥΪͬ���칹�壬�����ܺ��������Ʒ�Ӧ�����ʵĽṹ��ʽ_____________��
��6��HOCH2CH2OH�ܱ����Ը������������E��д��E��HOCH2CH2OH���ɸ߾���Ļ�ѧ����ʽ______________________��
���𰸡� ![]() 1,2-�������� �ǻ� ȡ����Ӧ �ӳɷ�Ӧ 2CH3CH2OH+O2��2CH3CHO+2H2O HOCH2CH2OH+2CH3COOH
1,2-�������� �ǻ� ȡ����Ӧ �ӳɷ�Ӧ 2CH3CH2OH+O2��2CH3CHO+2H2O HOCH2CH2OH+2CH3COOH ![]() H2O+CH3COOCH2CH2OOCCH3 CH3CH2CH2COOH��(CH3)2CHCOOH��HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3
H2O+CH3COOCH2CH2OOCCH3 CH3CH2CH2COOH��(CH3)2CHCOOH��HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3 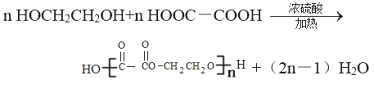
����������1����ϩ�Ľṹ��ʽΪCH2=CH2�������ʽΪ��![]() ����Ӧ�ٷ����ӳɷ�Ӧ��A�Ľṹ��ʽΪBrCH2CH2Br��������1��2-�������飻��2����ϩ����̼̼˫����ˮ�ӳ�����B���Ҵ������еĹ��������ǻ�����3����Ӧ����±������ˮ�ⷴӦ����Ӧ�۷����ӳɷ�Ӧ����4��CH3CH2OH��ͭ�������£�����������������Ӧ������CH3CHO����ȩ�����������ᣬ��Ӧ����������Ӧ��ȡ����Ӧ������ʽ�ֱ���2CH3CH2OH+O2
����Ӧ�ٷ����ӳɷ�Ӧ��A�Ľṹ��ʽΪBrCH2CH2Br��������1��2-�������飻��2����ϩ����̼̼˫����ˮ�ӳ�����B���Ҵ������еĹ��������ǻ�����3����Ӧ����±������ˮ�ⷴӦ����Ӧ�۷����ӳɷ�Ӧ����4��CH3CH2OH��ͭ�������£�����������������Ӧ������CH3CHO����ȩ�����������ᣬ��Ӧ����������Ӧ��ȡ����Ӧ������ʽ�ֱ���2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3COOH��HOCH2CH2OH
2CH3CHO+2H2O��2CH3COOH��HOCH2CH2OH![]() CH3COOCH2CH2OOCCH3��2H2O����5��B��D��Ӧ�������������������������ܺ��������Ʒ�Ӧ�����ʿ�������������࣬����ΪCH3CH2CH2COOH��(CH3)2CHCOOH��������HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3����6��HOCH2CH2OH�ܱ����Ը������������E��E��HOCH2CH2OH���ɸ߾��˵��E���Ҷ��ᣬ��Ӧ�Ļ�ѧ����ʽΪ
CH3COOCH2CH2OOCCH3��2H2O����5��B��D��Ӧ�������������������������ܺ��������Ʒ�Ӧ�����ʿ�������������࣬����ΪCH3CH2CH2COOH��(CH3)2CHCOOH��������HCOOCH2CH2CH3��HCOOCH(CH3)2��CH3CH2COOCH3����6��HOCH2CH2OH�ܱ����Ը������������E��E��HOCH2CH2OH���ɸ߾��˵��E���Ҷ��ᣬ��Ӧ�Ļ�ѧ����ʽΪ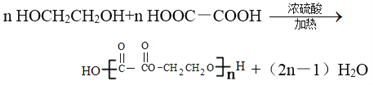 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�