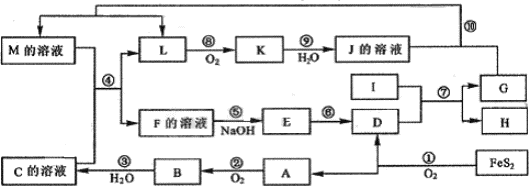
��Ŀ����
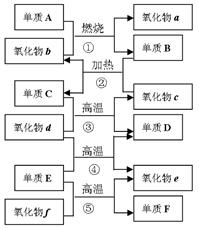
��15�֣�����ͼ��A��F����ѧ��ѧ����������,��A�pB�pE�pF��������ͬһ�ֽ���Ԫ�أ�G��һ�ֳ������ʡ�
��A�����壩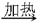 B + C + D ��C + E �� B + G; �� D + E �� F + G
B + C + D ��C + E �� B + G; �� D + E �� F + G
��A����Һ��+F����Һ����B����Һ��+D ��B����Һ�� + C + D �� A����Һ��
��1��д��A�pB�pC�pE�pF�Ļ�ѧʽ��A__________ B_________ C__________
E__________ F__________��5�֣�
��2��д����Ӧ�٢ڢܢݵĻ�ѧ����ʽ��
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��3����д��������E��������Ҫ��;��____________________________��2�֣�
��A�����壩
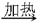 B + C + D ��C + E �� B + G; �� D + E �� F + G
B + C + D ��C + E �� B + G; �� D + E �� F + G��A����Һ��+F����Һ����B����Һ��+D ��B����Һ�� + C + D �� A����Һ��
��1��д��A�pB�pC�pE�pF�Ļ�ѧʽ��A__________ B_________ C__________
E__________ F__________��5�֣�
��2��д����Ӧ�٢ڢܢݵĻ�ѧ����ʽ��
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��_________________________________________________��2�֣�
��3����д��������E��������Ҫ��;��____________________________��2�֣�
��1��A: NaHCO3 B:Na2CO3 C:CO2 E:Na2O2 F:NaOH��5�֣�
��2����2NaHCO3=" Na2CO3" + CO2��+H2O��2�֣�
�� 2CO2 + 2Na2O2=" Na2CO3" +O2 ��2�֣�
��NaHCO3+ NaOH =" Na2CO3" +H2O��2�֣�
��Na2CO3 +CO2+H2O ="2NaHCO3 " ��2�֣�
��3���������pƯ����������������2�֣�
��2����2NaHCO3=" Na2CO3" + CO2��+H2O��2�֣�
�� 2CO2 + 2Na2O2=" Na2CO3" +O2 ��2�֣�
��NaHCO3+ NaOH =" Na2CO3" +H2O��2�֣�
��Na2CO3 +CO2+H2O ="2NaHCO3 " ��2�֣�
��3���������pƯ����������������2�֣�
��

��ϰ��ϵ�д�
�����Ŀ