��Ŀ����
(16��)��֪��RCH��CH2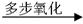 RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��
RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��

���У�X��Y��Ϊͬ���칹�壬F��C��Ϊͬϵ�E��D��Ϊͬϵ�B�к�֧�����Һ˴Ź������ײ����3�����շ壬���6��1��1��A���ܶ��ڱ�״����Ϊ1.25g/L��A��B��X��Y�ķ���ʽ������CnH2nO0. 5n-1
��1��B�Ľṹ��ʽΪ ��Y�Ľṹ��ʽΪ
��2��C��D����X�Ļ�ѧ��Ӧ����Ϊ
A���ӳɷ�Ӧ B����ȥ��Ӧ
C��ȡ����Ӧ D��������Ӧ
��3��ʵ������ȡA�Ļ�ѧ��Ӧ����ʽΪ ��
��4��ʵ������װA�ķ���װ��ʱ���õ��IJ���������Ҫ�оƾ��ơ�������
��5���ռ�A��װ���� (�����)
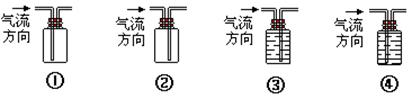
��6��ʵ������ȡAʱ�������¶ȹ��߶������д̼�����ζ������e�����һ����ʵ������֤����װ�������ɵ�A�л�ԭ�ԣ���ʹ������������ͨ��������Һ�е�һ�ֻ��֣�����ѡ�Լ���˳��Ϊ (����ţ��ɲ�ȫѡ��Ҳ�����ظ�ʹ��)
��Ʒ����Һ ����ˮ
������������Һ �����Ը��������Һ
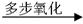 RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��
RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��
���У�X��Y��Ϊͬ���칹�壬F��C��Ϊͬϵ�E��D��Ϊͬϵ�B�к�֧�����Һ˴Ź������ײ����3�����շ壬���6��1��1��A���ܶ��ڱ�״����Ϊ1.25g/L��A��B��X��Y�ķ���ʽ������CnH2nO0. 5n-1
��1��B�Ľṹ��ʽΪ ��Y�Ľṹ��ʽΪ
��2��C��D����X�Ļ�ѧ��Ӧ����Ϊ
A���ӳɷ�Ӧ B����ȥ��Ӧ
C��ȡ����Ӧ D��������Ӧ
��3��ʵ������ȡA�Ļ�ѧ��Ӧ����ʽΪ ��
��4��ʵ������װA�ķ���װ��ʱ���õ��IJ���������Ҫ�оƾ��ơ�������
��5���ռ�A��װ���� (�����)

��6��ʵ������ȡAʱ�������¶ȹ��߶������д̼�����ζ������e�����һ����ʵ������֤����װ�������ɵ�A�л�ԭ�ԣ���ʹ������������ͨ��������Һ�е�һ�ֻ��֣�����ѡ�Լ���˳��Ϊ (����ţ��ɲ�ȫѡ��Ҳ�����ظ�ʹ��)
��Ʒ����Һ ����ˮ
������������Һ �����Ը��������Һ
��1��(CH3)2CHCHO �� CH3COOCH2CH(CH3)2����2�� CD ��
��3��CH3CH2OH CH2=CH2�� + H2O ��
CH2=CH2�� + H2O ��
��4����ƿ���¶ȼ� ����5�� �� ����6�� �ۢ٢� ��
˵������3������6����3�֣������2��
��3��CH3CH2OH
 CH2=CH2�� + H2O ��
CH2=CH2�� + H2O ����4����ƿ���¶ȼ� ����5�� �� ����6�� �ۢ٢� ��
˵������3������6����3�֣������2��
��

��ϰ��ϵ�д�
�����Ŀ

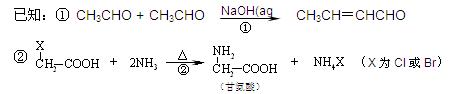
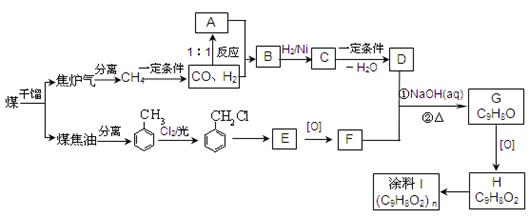
 H�ĺϳ�·�߲�ע����Ӧ������
H�ĺϳ�·�߲�ע����Ӧ������

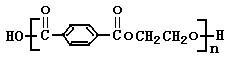
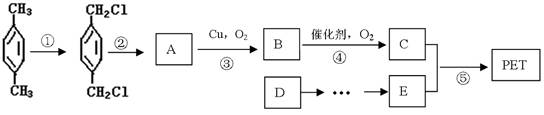
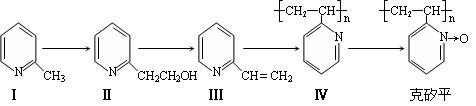
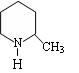
 ȷ���� ������ĸ��
ȷ���� ������ĸ��
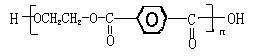 ������A��B���ֵ������۶��ɡ���֪�ױ����ڸ�����ش����������ɱ����ᡣ��Ҫ�Զ��ױ�����ϩΪԭ�ϣ����Լ�����ѡ�ϳɾ�����ά(��ȷ��)��
������A��B���ֵ������۶��ɡ���֪�ױ����ڸ�����ش����������ɱ����ᡣ��Ҫ�Զ��ױ�����ϩΪԭ�ϣ����Լ�����ѡ�ϳɾ�����ά(��ȷ��)��