��Ŀ����
��2012?�Ϻ�ģ�⣩ij��Ӧ���У������������������̡�������O2��NH4+������NO3-����������ǹ���������ϸ������ֳ���侻��Ӧ����ʽ��ʾ��
���������Ӧ�����ӷ���ʽ����ƽ����ϵ�����뷽���У���������ӵ�ת�Ʒ������Ŀ��
��1����Ӧ�У���ԭ����
��2�����̬���е�lmg��ת����������еĵ���������
��3��ȡ100mL��ȫ��Ӧ�Ժ����Һ������19.2gͭ�ۼ�һ������ϡ���ᣬǡ����ȫ��Ӧ�����軹ԭ����ȫ����NO���壩����ԭ��Һ��NH4+��Ũ��Ϊ
NH4+
NH4+
+2O2
2O2
| ����ϸ�� |
NO3-
NO3-
+2H+
2H+
+H2O
H2O
���������Ӧ�����ӷ���ʽ����ƽ����ϵ�����뷽���У���������ӵ�ת�Ʒ������Ŀ��
��1����Ӧ�У���ԭ����
NH4+
NH4+
������ԭ��Ԫ����O2�е�O
O2�е�O
����2�����̬���е�lmg��ת����������еĵ���������
4.57
4.57
mg������ȷ��0.01����3��ȡ100mL��ȫ��Ӧ�Ժ����Һ������19.2gͭ�ۼ�һ������ϡ���ᣬǡ����ȫ��Ӧ�����軹ԭ����ȫ����NO���壩����ԭ��Һ��NH4+��Ũ��Ϊ
2
2
mol/L�������跴Ӧǰ����Һ��������䣩����������Ϣ��֪��O2��NH4+����ΪNO3-�����ݵ���غ��֪��������H+����Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ����ϼ��ܹ�����8�ۣ�O2��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1��
��1��Ԫ�صĻ��ϼ�����Ϊ��ԭ����Ԫ�صĻ��ϼ۽��ͱ���ԭ���Դ������
��2���̬���е�1mg������NH4+������Ϊ1mg��
�����ݣ�1�������ӷ���ʽ������Ҫ������������
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��������NԪ���غ���n��NH4+��=n��NO3-�����ٸ���c=
���㣻
| -2 |
| O |
��1��Ԫ�صĻ��ϼ�����Ϊ��ԭ����Ԫ�صĻ��ϼ۽��ͱ���ԭ���Դ������
��2���̬���е�1mg������NH4+������Ϊ1mg��
| 18 |
| 14 |
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��������NԪ���غ���n��NH4+��=n��NO3-�����ٸ���c=
| n |
| V |
����⣺����Ϣ��֪��O2��NH4+����ΪNO3-�����ݵ���غ��֪��������H+����Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ����ϼ��ܹ�����8�ۣ�O2��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ����ϼ��ܹ�����4�ۣ����ݻ��ϼ�������ȿ�֪��NH4+ϵ��Ϊ1��O2ϵ��Ϊ2������NԪ���ǿ�֪NO3-ϵ��Ϊ1�����ݵ���غ��֪H+ϵ��Ϊ2������Ԫ���غ��֪������H2O����ϵ��Ϊ1���������ӷ���ʽΪNH4++2O2=NO3-+2H++H2O��
�ʴ�Ϊ��1NH4+��2O2��1NO3-��2H+��1H2O��
��1����������ԭ��Ӧ�У����ϼ����ߵ�Ԫ�ر���������������Ӧ��Ԫ�ػ��ϼ۽��͵�Ԫ�ر���ԭ���÷�Ӧ�з�Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ�NH4+Ϊ��ԭ����O2��
��OԪ�ػ��ϼ���0�۽���Ϊ-2�ۣ�����ԭ��Ԫ����O2�е�O�������ɵ�Ԫ��ת�Ƹ���Ԫ�أ�ת��8�����ӣ�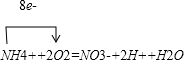
�ʴ�Ϊ��NH4+��O2�е�O��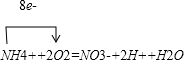 ��
��
��2������Ҫ����������Ϊx����
NH4++2O2=NO3-+2H++H2O��
18g 64g
1mg��
x
����x=
��1mg��
=4.57mg
�ʴ�Ϊ��4.57��
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��=
��2=0.6mol������n����NO3-��=0.2mol������NԪ���غ���n��NH4+��=n��NO3-��=0.2mol������ԭ��Һ��NH4+��Ũ��Ϊ
=2mol/L��
�ʴ�Ϊ��2��
| -2 |
| O |
�ʴ�Ϊ��1NH4+��2O2��1NO3-��2H+��1H2O��
��1����������ԭ��Ӧ�У����ϼ����ߵ�Ԫ�ر���������������Ӧ��Ԫ�ػ��ϼ۽��͵�Ԫ�ر���ԭ���÷�Ӧ�з�Ӧ��NH4+��NO3-��NԪ�ػ��ϼ���-3������Ϊ+5�ۣ�NH4+Ϊ��ԭ����O2��
| -2 |
| O |
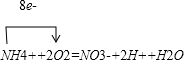
�ʴ�Ϊ��NH4+��O2�е�O��
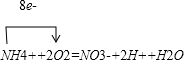 ��
����2������Ҫ����������Ϊx����
NH4++2O2=NO3-+2H++H2O��
18g 64g
1mg��
| 18 |
| 14 |
����x=
| 64g |
| 18g |
| 18 |
| 14 |
�ʴ�Ϊ��4.57��
��3����Ӧ��NO3-��NO��NԪ�ػ��ϼ���+5�۽���Ϊ+2�ۣ�Cu��Cu2+��CuԪ�ػ��ϼ���0������Ϊ+2�ۣ����ݵ���ת���غ���2n��Cu��=3n��NO3-��=
| 19.2g |
| 64g/mol |
| 0.2mol |
| 0.1L |
�ʴ�Ϊ��2��
���������⿼��������ԭ��Ӧ����ƽ�����ݷ���ʽ�ļ��㡢���ʵ���Ũ�ȼ���ȣ������غ��ж������������ǽ���Ĺؼ������ջ��ϼ���������ƽ������ԭ��Ӧ����ʽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2012?�Ϻ�ģ�⣩����ɫ��ѧʵ�顱�����ã�ij��ѧ��ʦΪ������������Ʒ�Ӧ�����������װ��������������صĿα�ʵ�飮ʵ�����������������Ӧ��װ�ÿ�����ͼ�Ľ�����һ���������������������������������ڷ�һ��ƶ�����Ľ����ƣ�������ú�ͣ���������β����һ�Ž���NaOH��Һ�������ȸ���Ԥ�ȣ��������ڳ�Բ��ʱ������ͨ�����������ɼ����Ż�ȼ�գ����ɴ������̣���������������ǣ�������
��2012?�Ϻ�ģ�⣩����ɫ��ѧʵ�顱�����ã�ij��ѧ��ʦΪ������������Ʒ�Ӧ�����������װ��������������صĿα�ʵ�飮ʵ�����������������Ӧ��װ�ÿ�����ͼ�Ľ�����һ���������������������������������ڷ�һ��ƶ�����Ľ����ƣ�������ú�ͣ���������β����һ�Ž���NaOH��Һ�������ȸ���Ԥ�ȣ��������ڳ�Բ��ʱ������ͨ�����������ɼ����Ż�ȼ�գ����ɴ������̣���������������ǣ�������