��Ŀ����
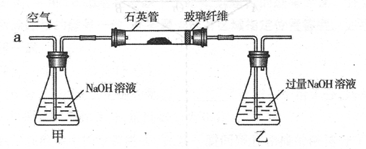
ij��ȤС���������ͼʵ��װ�ý���ʵ�顣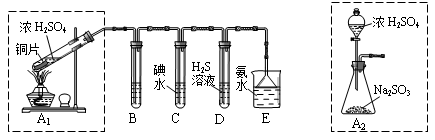
��̽��������Ⱦ��SO2������
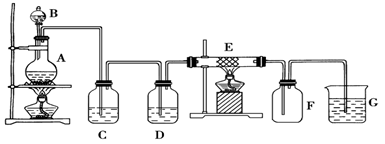
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�
Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽ
Ϊ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ�����
�ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�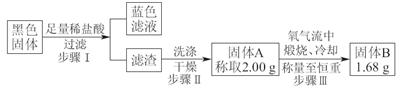
��3������� �м�������ϴ�Ӹɾ���ʵ�鷽����____________________________��
��4����ɫ����ijɷ���________________��
�������
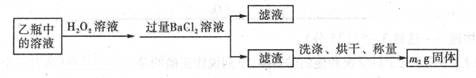
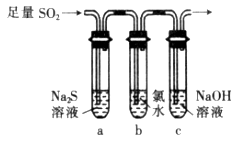
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���Ϊ ��
��1���ܣ�1�֣�
��2��Ʒ����Һ��1�֣� SO2��I2��2H2O��SO42-��2 I-��4H+��2�֣� SO2��2H2S="3S��+" 2H2O��2�֣�
��3��ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ����������������ϴ�Ӹɾ���2�֣���
��4��CuO��CuS��Cu2S��2�֣�
��5��NH3��H2O��SO2��NH4+��HSO3-��2�֣�
���������������1��A2װ�ò���Ҫ���ȣ��ҿ��Կ��Ƽ���Ũ����������Ʒ�Ӧ���У�����������2������SO2Ư����һ����Ʒ���Լ���C��SO2��ⵥ�ʷ�Ӧ��D����H2S��Ӧ���з�Ӧ����3��ȡ���һ��ϴ��Һ�������μ�����AgNO3��Һ����������������ϴ�Ӹɾ���ǿ�����һ��ϴ��Һ����4�����ݲ���������ɫ��Һ�ó����庬��CuO������������պ������ı仯���㣺��ȫΪCuS�����õ�����������ӦΪ2��96��2��144=1��5g����ȫΪCu2S�����õ�����������ӦΪ2��160��144=1��8g�����������ʾ����С���5������SO2Ӧ������������李�
���㣺����ʵ�鼰ʵ��������й����⡣

��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶá���֪��Na2S2O3��������Һ�в����ȶ����ڡ�
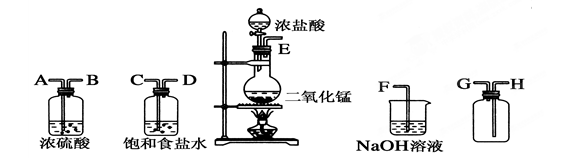
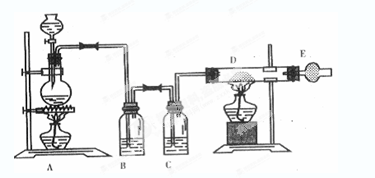
��1��ij�о�С����Ƶ��Ʊ�Na2S2O3��5H2O��װ�úͲ��ֲ����������¡�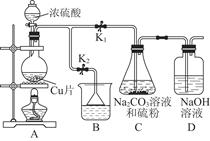
��.��K1�ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȡ�
��.C�л��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH �ӽ�7ʱ����K2���ر�K1��ֹͣC�еķ�Ӧ��ֹͣ���ȡ�
��.����C�еĻ��Һ��
��.����Һ���� �� �����ˡ�ϴ�ӡ���ɣ��õ���ƷNa2S2O3��5H2O��
�٢��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ���� �������ӷ���ʽ��ʾ����
�ڢ��У����������� �� ��
��װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ�� ��Һ��
����һС����ʵ���з��֣�����������������������º����������ԣ����ֲ��������������⣬�����Ʋ���ܵ�ԭ�� ��
��2������Na2S2O3��Һ�ⶨ��ˮ��Ba2����Ũ�ȣ��������£�ȡ��ˮ25.00 mL�������ʵ�����ȼ������� K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ�KI��Һ����ַ�Ӧ��û����ҺV mL������ƽ���ֳ�4�ȷݣ����������Һ��ָʾ������0.001 0 mol��L��1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ��������ݼ�¼���±���ʾ��
| ��� | 1 | 2 | 3 | 4 |
| ����Na2S2O3�� | | | | |
| ��Һ�����/mL | 18.02 | 17.98 | 18.00 | 20.03 |
���ַ�Ӧ���ӷ���ʽΪ��
��Cr2O72-��6I����14H��=2Cr3����3I2��7H2O��
��I2��2S2O32-=2I����S4O62-��
���жϴﵽ�ζ��յ�������� ����ˮ��Ba2�������ʵ���Ũ�� _��


 5CaCl2 + Ca(ClO3)2 + 6H2O
5CaCl2 + Ca(ClO3)2 + 6H2O

 = ��
= ��
 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2