��Ŀ����
����ѡһ���л���ѧ������
���������Ծ�������PTT��Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��
���������Ծ�������PTT��Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��
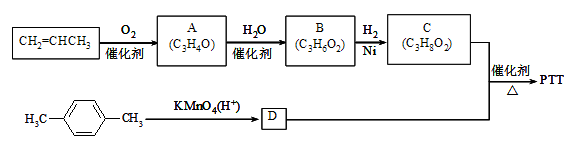
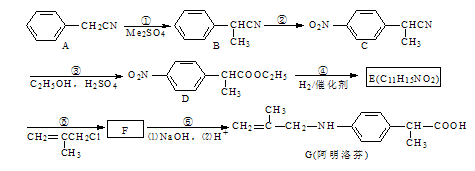
����A��B��C��Ϊ��״�����A�ܷ���������Ӧ��C�в�������1mol C���������Ʒ�Ӧ����22.4 L H2����״��������ش��������⣺
��1��A�����������ŵ�����Ϊ____________��
��2��B�Ľṹ��ʽΪ____________��A��B�ķ�Ӧ����Ϊ________________��
��3�� C��D��Ӧ����PTT�Ļ�ѧ����ʽΪ _______________��
��4������ʽΪC4H6O��A��Ϊͬϵ���ͬ���칹����____________�֡�
��1��A�����������ŵ�����Ϊ____________��
��2��B�Ľṹ��ʽΪ____________��A��B�ķ�Ӧ����Ϊ________________��
��3�� C��D��Ӧ����PTT�Ļ�ѧ����ʽΪ _______________��
��4������ʽΪC4H6O��A��Ϊͬϵ���ͬ���칹����____________�֡�
��1��̼̼˫�� �� ȩ��
��2��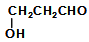 �� �ӳɷ�Ӧ
�� �ӳɷ�Ӧ
��3��

��4��3
��2��
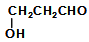 �� �ӳɷ�Ӧ
�� �ӳɷ�Ӧ ��3��


��4��3

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
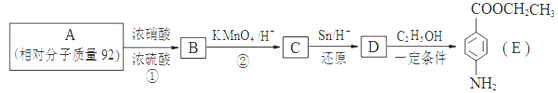
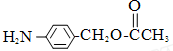 ��
��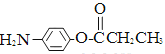 ��
��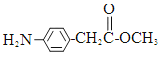 �⣬�������������Ļ�����E��ͬ���칹����___________��
�⣬�������������Ļ�����E��ͬ���칹����___________�� 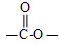 �ṹ�Ļ���
�ṹ�Ļ��� 
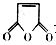 �Ǻϳ�ijЩҩ����м��塣����ƺ��������ɻ�����
�Ǻϳ�ijЩҩ����м��塣����ƺ��������ɻ�����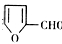 �ϳ�
�ϳ�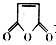 ��
��
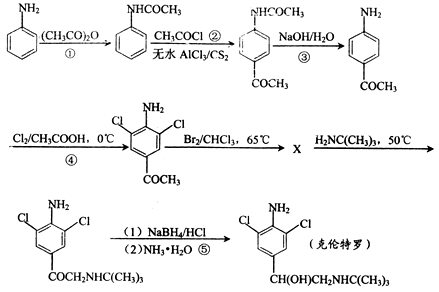
 ��ͬ���칹���ж��֣���д������������������������һ��___________
��ͬ���칹���ж��֣���д������������������������һ��___________