��Ŀ����
(14��)X��Y��Z��W��G����H���ɶ�����Ԫ����ɣ���������ѧ��ѧ�г��������壬�����������ʣ�
��X��Y��G��ʹʪ�����ɫʯ����ֽ��죬H��ʹʪ��ĺ�ɫʯ����ֽ������Z��W����ʹʪ���ʯ����ֽ��ɫ��
��X��H�����������̣�
��Y���γ��������Ҫ��������ʹƷ����Һ��ɫ��
��Z��W�������ɺ���ɫ���壻
��G��W��ȼ�տ��Բ���Y��H2O��
�ش��������⣺
��1�� H�Ļ�ѧʽ��__________��
ʵ������ȡH�Ļ�ѧ��Ӧ����ʽ��___________________________________________��
��2��Z�Ļ�ѧʽ��________��W�Ļ�ѧʽ��__________________��
��3�����з�����Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��4��ʵ�����Ʊ����ռ������Y���壬�����������¡�װ��A����Y���壬�������������Ӹ������ӿڣ�˳��Ϊa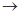
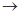
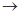
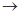
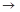 f��
f��
װ��D��������_ ___��װ��E��NaOH��Һ��������__ ____��
(1)NH3����1�֣�2NH4Cl+Ca(OH)2 CaCl2+2NH3��+H2O��2�֣�
CaCl2+2NH3��+H2O��2�֣�
(2) NO��1�֣���O2��1�֣� (3) 2H2S��3O2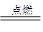 2H2O��2SO2��2�֣�
2H2O��2SO2��2�֣�
(4)d e c b����3�֣���ȫƿ����ֹ��������2�֣�β����������ֹ��Ⱦ������2�֣�
����

(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ԭ�������������Ǵ��������� |
| Z | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
 ��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣�� X��Y�е縺�Խ�ǿ����(��Ԫ�ط��ţ� ��XY2�ĵ���ʽ�� �������д��� ���Ҽ���
��Z2Y2�к��еĻ�ѧ�������� �����������ӵĸ�����Ϊ ��
��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
�ɷϾ�ӡˢ��·������W�ĵ���A����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·���ϵ�A����֪��
A(s)+H2SO4(aq) ="=" ASO4(aq) + H2(g)�� ��H=+64.4kJ��mol-1
2H2O2(l) ="=" 2H2O(l) + O2(g)�� ��H= -196.4kJ��mol-1
H2(g)+
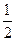 O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1��д��A��H2SO4��H2O2��Ӧ����ASO4(aq)��H2O(l)���Ȼ�ѧ����ʽ(A�û�ѧʽ��ʾ)��
�� ����
(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
|
Y |
ԭ�������������Ǵ��������� |
|
Z |
���ʼ��仯�������ɫ��ӦΪ��ɫ |
|
W |
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
�� Xλ��Ԫ�����ڱ��� �塣X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
�� X��Y�е縺�Խ�ǿ����(��Ԫ�ط��ţ� ��XY2�ĵ���ʽ�� �������д��� ���Ҽ���
��Z2Y2�к��еĻ�ѧ�������� �����������ӵĸ�����Ϊ ��
��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
�ɷϾ�ӡˢ��·������W�ĵ���A����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·���ϵ�A����֪��
A(s)+H2SO4 (aq) == ASO4(aq) + H2(g)�� ��H=+64.4kJ��mol-1
2H2O2(l) == 2H2O(l) + O2(g)�� ��H= -196.4kJ��mol-1
H2(g)+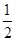 O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
��д��A��H2SO4��H2O2��Ӧ����ASO4(aq)��H2O(l)���Ȼ�ѧ����ʽ(A�û�ѧʽ��ʾ)��
�� ����
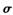 ����
����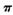 ������֮��Ϊ____________��
������֮��Ϊ____________��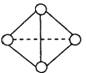
 Y�ų�942KJ��������д����Y4��̬���ӱ��Y2��̬���ӵ��Ȼ�ѧ����ʽ________________________
Y�ų�942KJ��������д����Y4��̬���ӱ��Y2��̬���ӵ��Ȼ�ѧ����ʽ________________________