��Ŀ����
(1)������Ԫ���������������۵ľ���ֵ��ȣ���������ɰ���Ԫ�ط������ڱ��еĢ�A�塣��ô����NaH�Ĵ��ڣ��ֿɰ���Ԫ�ط���________________�塣(2)ijԪ��X�ĺ�����������ں�����������ȡ��Ԫ�ص���
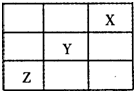
(3)������Ԫ��X��Y��Z�����ڱ��е�λ�ù�ϵ����ͼ��ʾ����

��Y��ZԪ�ص���̬�⻯����ȶ���_______________��_______________��(�ѧʽ)
����YԪ��ͬ���������ڵ�Ԫ�صĵ�����NaOH��Һ��Ӧ�����ӷ���ʽΪ______________��
������(1)����NaH��H�Ļ��ϼ�Ϊ-1������ɰ���Ԫ�ط��ڢ�A�塣
(2)ͨ������֪
n(O2)=![]() =0.1 mol
=0.1 mol
��ͨ��XO2��֪��X��O2�����ʵ�����Ӧ��
n(X)=n(O2)=0.1 mol
M(X)=![]() =
=
����Ϊ������������ں�����������
���������Ϊ14����Ϊ��Ԫ�ء�
(3)ͨ��Ԫ�����ڱ��е�λ�ã����ƶϳ�X��He��Y��F��Z��S��
������⻯����ȶ���HF��H2S��
����YԪ��ͬ���������ڵ�Ԫ�ص���ΪCl2������NaOH��Ӧ�����ӷ���ʽΪCl2+2OH-![]() ClO-+Cl-+H2O��
ClO-+Cl-+H2O��
�𰸣�(1)��A��
(2)��2���ڢ�A��
(3)��HF H2S ��Cl2+2OH-![]() Cl-+ClO-+H2O
Cl-+ClO-+H2O

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��1��������Ԫ���������������۵ľ���ֵ��ȣ���������ɰ���Ԫ�ط������ڱ��еĢ�A�壮��ô����NaH�Ĵ��ڣ��ֿɰ���Ԫ�ط���
��1��������Ԫ���������������۵ľ���ֵ��ȣ���������ɰ���Ԫ�ط������ڱ��еĢ�A�壮��ô����NaH�Ĵ��ڣ��ֿɰ���Ԫ�ط���

