��Ŀ����
�����仯���������ǵijԡ�����ס���С������ȶ��������е���ϵ��Ҳ�Ǹ��л�ѧѧϰ����Ҫ��һ���֣���ش��������⣺
��1��һ�������£���һ��2L���ܱ������г���2mo1N2��6molH2����Ӧ��ƽ��ʱ�ų�93kJ����������NH3��Ũ��Ϊ1mol/L����д���÷�Ӧ���Ȼ�ѧ����ʽ______��
��2������һ֧l0mL���Թܣ�����NO������ˮ���У����Թ��л���ͨA-�������������Թ���Һ���ȶ�ʱ��ʣ������2mL����ͨ���������������Ϊ______��
��3��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2g��?4NO��g��+6H2O��g����
| ��ʼŨ�ȣ�mol�� L-1�� | c�� NH3�� | ��c�� O2�� | c�� NO�� | c�� H20�� |
| �� | 1 | 2 | 0 | 0 |
| �� | 2 | 4 | 0 | 0 |
| �� | 0.5 | x | y | z |
�ں��º����£���Ҫʹ�����ƽ��ʱ�����Ũ����ͬ����x=______��y=______��z=______��
��4�����ݻ���ͬ���¶ȷֱ�ΪT1��T2�������ܱ������зֱ�������NO2��������Ӧ��2NO2��g��?N2O4��g����H��0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1��T2����a1______a2��
A�����ڡ���B��С�ڡ���c�����ڡ���D�����϶��п���
��5����״���£������﴿���İ��Ͷ�����������ֱ������Ȫʵ���������Һ�������ϣ���Ӧ����Һ�е�����Ũ�ȹ�ϵ��ȷ����______��
A��c��NO-3����c��NH+4����c��H+����c��OH-��
B��c��NH+4����c��NO-3����c��OH-����c��H+��
C��c��H+��=c��OH-��+c��NH3��H2O��
D��c��NH4+��+c��NH3��H2O��=1.5c��NO-3��
���Ȼ�ѧ��Ӧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-93kJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-93kJ/mol��
��2����4NO+3O2+2H2O�T4HNO3��֪����ʣ������ΪNO��������Ϊ��10mL-2mL����
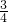 =6mL��
=6mL����ʣ������Ϊ��������10mLNO��ȫת��Ϊ���ᣬ����Ϊ7.5mL+2mL=9.5mL��
�ʴ�Ϊ��6mL��9.5mL��
��3�������е����ʵ����ȼĴ�ѹǿԽ��ѧƽ�������ƶ��ij̶ȴ����ҵ�ת����С���ʴ�Ϊ������
�ڸ��ݵ�Чƽ���֪������ת��Ϊ��ʼ������е���ʼ����ͬ����
4NH3��g��+5O2g��?4NO��g��+6H2O��g����
��ʼ1 2
�� 0.5 x y z
��0.5+y=1��0.5+
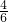 z=1��x+
z=1��x+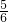 z=2�����x=1.375��y=0.5��z=0.75��
z=2�����x=1.375��y=0.5��z=0.75���ʴ�Ϊ��1.375��0.5��0.75��
��4�����º����·�Ӧ��ͬʱ�䣬T1��T2�������ܱ������зֱ�������NO2����pV=nRT��֪�����������е�ѹǿ���ʱ��
a1=a2��
��P1��P2��a1����a2��
��P1��P2��a1����a2�������϶��п��ܣ�
�ʴ�Ϊ��D��
��5�������Ȫʵ���������Һ�������Ϻ���������泥�NH4NO3�TNH4++NO3-��NH4++H2O?NH3��H2O+H+��
��c��NO-3����c��NH+4����c��H+����c��OH-������A��ȷ����B����
�������غ��֪��c��H+��=c��OH-��+c��NH3��H2O������C��ȷ���������غ��֪��c��NH4+��+c��NH3��H2O��=c��NO-3������D����
�ʴ�Ϊ��AC��
��������1��������NH3��Ũ��Ϊ1mol/L��������1mol/L��2L=2molNH3�ų�������Ϊ93kJ���Դ�����д�Ȼ�ѧ��Ӧ����ʽ��
��2������4NO+3O2+2H2O�T4HNO3��ʣ���������ΪNO��������������
��3�������е����ʵ����ȼĴ�����ѹǿ�Ի�ѧƽ���Ӱ����������
�ڸ��ݵ�Чƽ���֪������ת��Ϊ��ʼ������е���ʼ����ͬ��
��4�����º����·�Ӧ��ͬʱ�䣬T1��T2�������ܱ������зֱ�������NO2����pV=nRT��֪�����������е�ѹǿ������Ȼȣ�
��5�������Ȫʵ���������Һ�������Ϻ���������泥����õ��뼰ˮ����������
������������ѣ������Ȼ�ѧ��Ӧ����ʽ����ѧƽ�⡢���롢ˮ�⡢����Ũ�ȵıȽϡ�NO�Ļ�ѧ���ʵȣ��ۺ���ǿ����4����ѧ�������ѵ���״��㣮

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д���1��һ�������£���һ��2L���ܱ������г���2molN2��6molH2����Ӧ��ƽ��ʱ�ų�93kJ����������NH3��Ũ��Ϊ1mol/L����д���÷�Ӧ���Ȼ�ѧ����ʽ
��2������һ֧l0mL���Թܣ�����NO������ˮ���У����Թ��л���ͨA-�������������Թ���Һ���ȶ�ʱ��ʣ������2mL����ͨ���������������Ϊ
��3��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2g��?4NO��g��+6H2O��g����
| ��ʼŨ�ȣ�mol�� L-1�� | c�� NH3�� | c�� O2�� | c�� NO�� | c�� H20�� |
| �� | 1 | 2 | 0 | 0 |
| �� | 2 | 4 | 0 | 0 |
| �� | 0.5 | x | y | z |
�ں��º����£���Ҫʹ�����ƽ��ʱ�����Ũ����ͬ����x=
��4�����ݻ���ͬ���¶ȷֱ�ΪT1��T2�������ܱ������зֱ�������NO2��������Ӧ��2NO2��g��?N2O4��g����H��0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1��T2����a1
A������ B��С�� c������ D�����϶��п���
��5����״���£������﴿���İ��Ͷ�����������ֱ������Ȫʵ���������Һ�������ϣ���Ӧ����Һ�е�����Ũ�ȹ�ϵ��ȷ����
A��c��NO-3����c��NH+4����c��H+����c��OH-��
B��c��NH+4����c��NO-3����c��OH-����c��H+��
C��c��H+��=c��OH-��+c��NH3��H2O��
D��c��NH4+��+c��NH3��H2O��=1.5c��NO-3��
�����仯���������ǵijԡ�����ס���С������ȶ��������е���ϵ��Ҳ�Ǹ��л�ѧѧϰ����Ҫ��һ���֡���ش��������⣺
I����1������һ֧15mL���Թܣ�����NO������ˮ���У����Թ��л���ͨ��һ�������������Թ���Һ���ȶ�ʱ��ʣ������3mL����ͨ���������������Ϊ ��
 ��2��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2��g��
4NO��g��+6H2O��g����
��2��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2��g��
4NO��g��+6H2O��g����
|
��ʼŨ�ȣ� mol/L�� |
C(NH3) |
C(O2) |
C(NO) |
C(H2O) |
|
�� |
1 |
2 |
0 |
0 |
|
�� |
4 |
8 |
0 |
0 |
|
�� |
0.2 |
x |
y |
z |
�ٺ��º����£�ƽ��ʱNH3��ת���ʼ� �ҡ������������=����������
�ں��º����£���Ҫʹ�����ƽ��ʱ�����Ũ����ͬ����x= ��y= ��z= ��
��3�����ݻ���ͬ���¶ȷֱ�ΪT1��T2�������ܱ������зֱ�������NO2��������Ӧ��2NO2��g�� N2O4��g����H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1< T2����a1_
a2��
N2O4��g����H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1< T2����a1_
a2��
A������ B��С�� C������ D�����϶��п���
��4��2��24L����״����������200 mL l mol/L HNO3��Һ���պ�Ӧ����Һ�е�����Ũ�ȹ�ϵ�� ��
������������NF3����һ�����͵ĵ��Ӳ��ϣ����ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ������������HF�� NO�� HNO3������Ҫ��ش��������⣺
��1��д���÷�Ӧ�Ļ�ѧ����ʽ�� ����Ӧ�����У��������ͻ�ԭ�����ʵ���֮��Ϊ ��
��2������Ӧ������0��2mol HNO3��ת�Ƶĵ�����ĿΪ ��
 4NO(g)+6H2O(g)
4NO(g)+6H2O(g) 
 N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��
N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��