��Ŀ����
(12��)������һ���¶��µ�ijNaOH��Һ����֪:����Һ���ΪV mL����Һ�ܶ�Ϊd g/cm3����������(�����ٷֱ�Ũ��)Ϊw%�����ʵ���Ũ��Ϊc mol/L����Һ�к��������Ƶ�����Ϊm g. �Իش��������⣮
(1)���㣺��w��d��ʾ��Һ�����ʵ����ʵ���Ũ��cΪ________________��
(2)ijѧ����������ƽ����С�ձ�������(�ձ���ʢNaOH)������ǰ��������ڱ�ߵ���̶ȣ���ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�________________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________ ________________ ���ٶ����ճ���С�ձ�������Ϊ________________(�32.6 g����32.61 g��)��
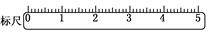
(3)�ڱ��(ͼ)�ϻ�������λ��(����������ʾ)��
(4)���ݺ���Һ���ȣ�����ʱ����Һ����ڿ̶��ߣ������ּ�����ˮ���̶��ߣ�������Һ�����ʵ���Ũ�Ƚ�__________________(�ƫ����ƫС������Ӱ�족)��
(1)���㣺��w��d��ʾ��Һ�����ʵ����ʵ���Ũ��cΪ________________��
(2)ijѧ����������ƽ����С�ձ�������(�ձ���ʢNaOH)������ǰ��������ڱ�ߵ���̶ȣ���ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�________________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________ ________________ ���ٶ����ճ���С�ձ�������Ϊ________________(�32.6 g����32.61 g��)��
(3)�ڱ��(ͼ)�ϻ�������λ��(����������ʾ)��

(4)���ݺ���Һ���ȣ�����ʱ����Һ����ڿ̶��ߣ������ּ�����ˮ���̶��ߣ�������Һ�����ʵ���Ũ�Ƚ�__________________(�ƫ����ƫС������Ӱ�족)��
(1)c��0.25dw mol/L��(2)���� ����������ҩƷ. 32.6 g
(3) ��4��ƫС (ÿ��2��)
��4��ƫС (ÿ��2��)
(3)
 ��4��ƫС (ÿ��2��)
��4��ƫС (ÿ��2��)(1)����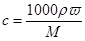 ��֪��
��֪��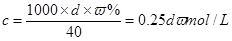 ��
��
��2��ָ���ڷֶ��̵�ƫ��λ�ã�˵���Ҷ�����������˵ģ������ߵ����̽������ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ӧ��������������ҩƷ��������ƽֻ�ܶ�����0.1g�����Դ�ѡ32.6 g��
��3�����������֪�������������2.6g�����Դ���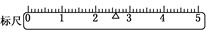 ��
��
��4���ּ�����ˮ���̶��ߣ�˵����Һ�����ƫ��Ũ��ƫС��
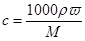 ��֪��
��֪��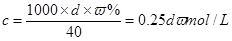 ��
����2��ָ���ڷֶ��̵�ƫ��λ�ã�˵���Ҷ�����������˵ģ������ߵ����̽������ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ӧ��������������ҩƷ��������ƽֻ�ܶ�����0.1g�����Դ�ѡ32.6 g��
��3�����������֪�������������2.6g�����Դ���
 ��
����4���ּ�����ˮ���̶��ߣ�˵����Һ�����ƫ��Ũ��ƫС��

��ϰ��ϵ�д�
�����Ŀ