��Ŀ����
��Fe3+��Fe2+������������ʽ������ȫʱ����Һ��pH�ֱ�Ϊ3.2��9.7��
��Ba(NO3)2����ķֽ��¶ȣ�592�棻
��Ksp(BaSO4)=1.1��10-10��Ksp(BaCO3)=5.1��10-9��

��2��������������ʱ��Ba(FeO2)2��HNO3��Ӧ�������������Σ���Ӧ�Ļ�ѧ����ʽΪ��______________________��
��3���ó���ϱ���ʵ�ʣ�ѡ�õ�XΪ________������ţ���
A��BaCl2 B��BaCO3 C��Ba(NO3)2 D��Ba(OH)2
��4���к�Iʹ��Һ��p HΪ4��5Ŀ����__________________��������ӷ���ʽ����ԭ��_______________________��
��5����Ba(NO3)2��Һ�л���侧��IJ���������___________________��
��6���ⶨ����Ba(NO3)2����Ĵ��ȣ�ȷ��ȡw�˾�����������ˮ���������������ᣬ��ַ�Ӧ���ˡ�ϴ�ӡ��������������Ϊm�ˣ���þ���Ĵ���Ϊ______________��
 BaCO3+ SO42��
BaCO3+ SO42����2��Ba(FeO2)2 +8HNO3 = Ba(NO3)2+2Fe(NO3)3+4H2O
��3��B
��4��ʹFe3+��ȫ������Fe3++3H2O
 Fe(OH)3+ H+��BaCO3����H+ʹFe3+ˮ��ƽ�����ƣ��γ�Fe(OH)3����
Fe(OH)3+ H+��BaCO3����H+ʹFe3+ˮ��ƽ�����ƣ��γ�Fe(OH)3������5�������ᾧ
��6��261m/233w��100%

��.ʵ�������CuO��H2��ԭʱҲ��Cu2O���ɡ���һ������H2����ͨ�����ȵ�CuO��ĩ���õ����������һ������m(Cu)��m(O)=8��a����a�в�ͬ��ȡֵʱ����������ɷֲ�ͬ�������a��ȡֵ��Χ��������ɷֵĹ�ϵ�������±�����һ������������Ҳ���������ӣ���
a��ȡֵ��Χ | ��Ӧ�����ijɷ֣��û�ѧʽ��ʾ�� |
|
|
|
|
|
|
��.��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ����2.12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05 mol��L-1��NaOH��Һ������һ����Һ�еμ�0.600 mol��L-1 Ba(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��
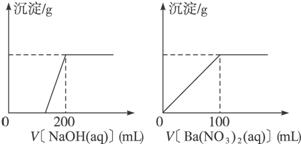
��1����ͨ������ȷ��m��ֵ��
��2��X��Ħ������Ϊ368 g��mol-1,��ȷ��X�Ļ�ѧʽ_______________
����A~D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ�Ӧ����ʽ��ʾ���ǣ� ��
|
|
I |
II |
|
A |
����CO2ͨ��NaOH��Һ�� |
����CO2ͨ��NaOH��Һ�� |
|
B |
����NaOH��Һ����Al2(SO4)3��Һ�� |
����Al2(SO4)3��Һ����Ũ��ˮ�� |
|
C |
0.1molCl2ͨ�뺬0.2molFeBr2����Һ�� |
0.3mol Cl2ͨ�뺬0.2mol FeBr2����Һ�� |
|
D |
����BaCl2��Һ������Na2SO4��Һ���� |
����Ba(NO3)2��Һ�����MgSO4��Һ���� |

 B����SO2ˮ��Һ�еμ������ữ��Ba(NO3)2��Һ���а�ɫ�������ɣ�˵��BaSO3����������
B����SO2ˮ��Һ�еμ������ữ��Ba(NO3)2��Һ���а�ɫ�������ɣ�˵��BaSO3����������