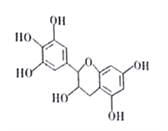
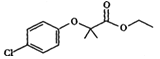
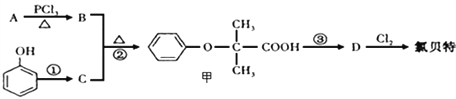
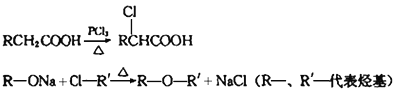̀âÄ¿ÄÚÈƯ
¡¾̀âÄ¿¡¿¶̀ÖÜÆÚÔªËØA¡¢B¡¢C¡¢D¡¢EµÄÔ×ÓĐ̣ÊửÀ´ÎÔö´ó£¬AºÍCͬ×壬BºÍDͬ×壬CÊÇËùÔÚÖÜÆÚÔ×Ӱ뾶×î´óµÄÖ÷×åÔªËØ£¬AºÍB¡¢D¡¢E¾ùÄÜĐγɹ²¼ÛĐÍ»¯ºÏÎï¡£AºÍB¡¢CºÍEĐγɵĻ¯ºÏÎïÔÚË®ÖĐ¾ù³Ê¼îĐÔ£®»Ø´đÏÂÁĐÎỀ⣺
£¨1£©ÔªËØDÔÚÖÜÆÚ±íÖеÄλÖĂ____________£¬ÆäÇ⻯ÎïµÄ½á¹¹Ê½ÊÇ___________£»
£¨2£©B¡¢C¡¢EÈưÖÖÔªËØĐγɵļ̣µ¥Àë×ӵİ뾶ÓÉ´óµ½Đ¡µÄ˳Đ̣ÊÇ£¨ÓĂÀë×Ó·ûºÅ±íʾ£©___________£»
£¨3£©A·Ö±đÓëB¡¢EĐγɵĻ¯ºÏÎï¿É̉ÔÏ໥·´Ó¦£¬Đ´³öÉú³ÉµÄƠưÑεĵç×Óʽ_____________£»
£¨4£©A¡¢BºÍE¸öÊư±ÈΪ5:1:1ĐγɵĻ¯ºÏÎïÖĐ´æÔڵĻ¯Ñ§¼üÀàĐÍΪ___________£¬ÆäÓë×ăÁ¿µÄÇâÑơ»¯ÄÆÈÜ̉º·´Ó¦µÄÀë×Ó·½³̀ʽΪ______________________________£»
£¨5£©CºÍEĐγɵĻ¯ºÏÎïÔÚË®ÖĐ³Ê¼îĐÔµÄỘ̉£¨ÓĂÀë×Ó·½³̀ʽ±íʾ)ÊÇ______________________¡£
¡¾´đ°¸¡¿ µÚÈưÖÜÆÚ¢ơA×å ![]() S2->N3->Na+
S2->N3->Na+ ![]() Àë×Ó¼ü¡¢¹²¼Û¼ü 2OH¡ª + HS-+NH4+ = S2-+NH3¡¤H2O+H2O»̣2OH¡ª + HS-+NH4+ = S2-+NH3+2H2O S2-+H2O
Àë×Ó¼ü¡¢¹²¼Û¼ü 2OH¡ª + HS-+NH4+ = S2-+NH3¡¤H2O+H2O»̣2OH¡ª + HS-+NH4+ = S2-+NH3+2H2O S2-+H2O![]() HS-+OH-
HS-+OH-
¡¾½âÎö¡¿ÊỒâ·ÖÎö£º¶̀ÖÜÆÚÔªËØA¡¢B¡¢C¡¢D¡¢EµÄÔ×ÓĐ̣ÊửÀ´ÎÔö´ó£¬AºÍCͬ×壬BºÍDͬ×壬CÊÇËùÔÚÖÜÆÚÔ×Ӱ뾶×î´óµÄÖ÷×åÔªËØ£¬ỘAΪH¡¢CΪNa¡£AºÍB¡¢D¡¢E¾ùÄÜĐγɹ²¼ÛĐÍ»¯ºÏÎï¡£AºÍB¡¢CºÍEĐγɵĻ¯ºÏÎïÔÚË®ÖĐ¾ù³Ê¼îĐÔ£¬ỘBΪN¡¢DΪP¡¢EΪS£®
£¨1£©ÔªËØDÔÚÖÜÆÚ±íÖеÄλÖõÚÈưÖÜÆÚ¢ơA×壬ÆäÇ⻯ÎïΪPH3£¬½á¹¹Ê½ÊÇ![]() £»
£»
£¨2£©B¡¢C¡¢EÈưÖÖÔªËØĐγɵļ̣µ¥Àë×ÓÖĐ£¬S2-ÓĐ3¸öµç×Ó²ă£¬N3-ºÍNa+¶¼ÓĐ2¸öµç×Ó²ă£¬µç×Ó²ă¶àµÄ°ë¾¶½Ï´ó£¬µç×Ó²ăÊưÏàͬµÄ£¬ºËµçºÉÊư½ÏĐ¡µÄ°ë¾¶´ó£¬Ëù̉԰뾶ÓÉ´óµ½Đ¡µÄ˳Đ̣ÊÇS2->N3->Na+£»
£¨3£©A·Ö±đÓëB¡¢EĐγɵĻ¯ºÏÎïÊÇ°±ÆøºÍẠ́»¯Ç⣬ËüĂÇ¿É̉ÔÏ໥·´Ó¦£¬Éú³ÉµÄƠưÑÎΪẠ́»¯ï§£¬Æäµç×Óʽ![]() £»
£»
£¨4£©A¡¢BºÍE¸öÊư±ÈΪ5:1:1ĐγɵĻ¯ºÏÎïÊÇNH4HS£¬ÆäÖĐ´æÔڵĻ¯Ñ§¼üΪÀë×Ó¼ü¡¢¹²¼Û¼ü£¬ÆäÓë×ăÁ¿µÄÇâÑơ»¯ÄÆÈÜ̉º·´Ó¦µÄÀë×Ó·½³̀ʽΪ2OH¡ª + HS-+NH4+ = S2-+NH3¡¤H2O+H2O»̣2OH¡ª + HS-+NH4+ = S2-+NH3+2H2O£»
£¨5£©CºÍEĐγɵĻ¯ºÏÎïẠ́»¯ÄÆÔÚË®ÖĐ³Ê¼îĐÔ£¬ÊÇ̣̉ΪẠ́Àë×ÓË®½âʹÈÜ̉º³Ê¼îĐÔ£¬ÆäÀë×Ó·½³̀ʽΪS2-+H2O![]() HS-+OH-¡£
HS-+OH-¡£

 ĂûĐ£¿Î̀ĂϵÁĐ´đ°¸
ĂûĐ£¿Î̀ĂϵÁĐ´đ°¸